Reported in @science.org, lithium pentasilacyclopentadienyl complexes.
These silicon analogues of the cyclopentadienyl ligand have a non-planar 5-membered ring and further characterisation shows evidence of aromaticity.
🔗 CSD Entry EXOMUD: dx.doi.org/10.5517/ccdc...
#FeaturedStructureFriday
20.02.2026 14:02 —
👍 23
🔁 7
💬 0
📌 0

The last work of Clément TANGUY and Emma Robert on the formal total synthesis of Strictamine is now published in @helvchimacta.bsky.social !
onlinelibrary.wiley.com/doi/10.1002/...
30.01.2026 14:20 —
👍 6
🔁 3
💬 0
📌 0

A Pd2L4-type receptor for squaraine dyes enabling ultrafast dark resonance energy transfer. Work by Damien Chen with help from Sybille Collignon, Farzaneh Fadaei-Tirani, and the @feldmannlab.bsky.social. Now out in @angewandtechemie.bsky.social: onlinelibrary.wiley.com/doi/10.1002/...
02.02.2026 09:29 —
👍 18
🔁 4
💬 0
📌 1

A new national research programme recognizes EPFL's expertise
The Swiss Confederation launches six new National Centres of Competence in Research (NCCRs). The NCCR “Separations”, which aims to accelerate research in separation sciences - the quest for chemical a...
We are delighted to announce the launch of NCCR Separations — a new National Center of Competence in Research dedicated to breakthrough separation technologies for a sustainable future!
Read more: actu.epfl.ch/news/a-new-n...
#NCCRSeparations #EPFL #GreenChemistry #EnergyTransition #ClimateTech
30.01.2026 16:45 —
👍 12
🔁 2
💬 0
📌 2

Start your independent as an ELISIR fellow right after your PhD, in one of the most terrific places in Europe !
14.01.2026 13:12 —
👍 16
🔁 11
💬 0
📌 1

Have a look at the last work of @duncanbrownsey.bsky.social and Alexandre Schoepfer combining small rings and Pd catalysis for the stereoselective synthesis of spirocyclic cyclopropane-oxazolines just published @chemicalscience.rsc.org !
pubs.rsc.org/en/Content/A...
07.01.2026 11:24 —
👍 11
🔁 3
💬 0
📌 1
A publication by the talented Atena Solea @atenasolea.bsky.social about ‘golden nano onions’ was selected as the ‘Paper of the Year 2025’
22.12.2025 07:45 —
👍 11
🔁 2
💬 0
📌 0

N-Heterocyclic vinylidenes: a new ligand class with great potential!
For a short write-up of first results, see: www.chimia.ch/chimia/artic...
#chemsky #EPFL #openaccess
19.12.2025 08:04 —
👍 12
🔁 2
💬 0
📌 0

This week I had the opportunity to present my results at the @rsc-masc.bsky.social meeting. It's hard to put into words the impression this meeting left on me, but imagine two intense days packed with inspiring science and even more amazing people. Thank you to the organizers!🙏🏻 #MASC2025 #RSC_MASC
17.12.2025 18:45 —
👍 7
🔁 2
💬 0
📌 0
I am very happy to announce that I’ve joined UVSQ @uvsq.bsky.social (Université Paris-Saclay) as a Junior Professor (Chaire de Professeur Junior).
03.12.2025 09:47 —
👍 10
🔁 1
💬 0
📌 0

Jean de Montmollin (@jcedemontmollin.bsky.social ) has destroyed coordination cages to examine their thermodynamic and kinetic stability. The results are summarized in an #openaccess publication in @daltontrans.rsc.org : pubs.rsc.org/en/content/a...
#chemsky #EPFL
10.10.2025 05:28 —
👍 17
🔁 4
💬 0
📌 1

Phosphanides are able to capture nitrous oxide (N2O, #laughinggas). Work by Alex Genoux (@alexgenouxchem.bsky.social ), Dicky Wong (@thwongax.bsky.social ), and Farzaneh Fadaei-Tirani. Now out (#openaccess ) in @chemcomm.rsc.org : pubs.rsc.org/en/content/a...
#chemsky #EPFL
12.09.2025 13:57 —
👍 26
🔁 4
💬 1
📌 0

OMCOS 22 is coming to an end, and the torch is being passed to the next OMCOS meeting. OMCOS 23 will be held in Lausanne and hosted by Professor Nicolai Cramer. #omcos22
05.09.2025 04:40 —
👍 8
🔁 2
💬 0
📌 0

Peptide/Protein Functionalization and Macrocyclization via Alkyne Umpolung with Hypervalent Iodine Reagents
ConspectusAlkynes are one of the most fundamental functional groups in organic synthesis due to the versatile chemistry of the triple bond, their unique rigid structure, and their use in bioconjugation. The introduction of alkynes onto organic molecules traditionally relies on nucleophilic activation, often requiring strong bases or metal catalysts. These conditions, however, restrict applications involving biomolecules such as peptides and proteins due to functional group incompatibility. To address this limitation, our group developed an “umpolung” approach, utilizing hypervalent iodine compounds to create electrophilic alkyne transfer reagents such as benziodoxol(on)es (Bx(X)s) and benziodazolones (BZs). The high reactivity of EBx/X/Z reagents enables efficient alkyne transfer to various nucleophilic residues in peptides and proteins under different reaction conditions, providing a versatile tool for biomolecule modification.In this Account, we highlight the residue-selective alkynylation and alkenylation of peptides enabled by the development of novel EBx/X/Z reagents with a focus on progress since 2021. This includes the following: (1) Selective residue modification: We have made significant progress in the residue-selective alkynylation and alkenylation of peptides and proteins. Building on our initial work with Cys-selective alkynylation, we enhanced reactivity and solubility by introducing a sulfonate group on the benziodoxolone arene core, facilitating lipophilic alkynylation in an aqueous environment. Furthermore, we developed perfluoroaryl-modified BZ reagents to achieve sequential Cys-Cys cross-linking and used them for antibody cross-linking with superior reactivity compared to that of conventional methods. Additionally, we expanded the reactivity beyond Cys to achieve Tyr-selective conjugation. All of these achievements underscored the tunability of EBx/X/Z reagents through strategic substituent modification on the iodine core. (2) Peptide stapling and macrocyclization: We designed EBx(X) reagents featuring an additional reactive site on the alkyne moiety, enabling Cys-Cys and Cys-Lys stapling in peptides. This approach enhanced their α-helicity and potential as PPI inhibitors with improved binding affinity to the MDM2 protein. For sequences lacking Cys, we incorporated the whole EBx(X) core onto Lys residues via an activated ester on the alkyne, forming peptide-EBx(X) conjugates. These conjugates facilitated the formation of rigid, functional peptide macrocycles using C-terminal or Trp-selective alkynylation. The utility of these macrocyclizations was demonstrated by achieving improved binding affinity to the KEAP1 protein and by generating fluorescent cyclic peptides suitable for live-cell imaging without additional fluorophores. (3) Broadening applicability with EBx-containing amino acids: We prepared EBx amino acids compatible with both solid-phase peptide synthesis (SPPS) and solution-phase synthesis (SPS), allowing us to apply our cyclization strategies to construct a diverse library of cyclic peptides.
The review of @xingyuliu9595.bsky.social on the group work on peptide/protein functionalization and macrocyclization has been published at Acc. Chem. Res.
doi.org/10.1021/acs....
What next? With all these nice tools developed, we are eager to collaborate with chemical biologists for applications!
30.08.2025 07:08 —
👍 9
🔁 2
💬 0
📌 0

📢pls share
We are hiring! New opening for a W2 Professor in "experimental inorganic chemistry" @unibonn.bsky.social
Deadline Oct. 10 t.co/EqMLA0fCuC
19.08.2025 20:09 —
👍 33
🔁 24
💬 3
📌 3
and Rosario Scopelliti (XRD)
13.08.2025 10:42 —
👍 2
🔁 0
💬 0
📌 0
With help of Renata Svecova (synthesis) and Farzaneh Fadaei-Tirani (XRD). Thanks a lot!!
13.08.2025 10:35 —
👍 2
🔁 0
💬 1
📌 0

Noga Eren has investigated the constitutional dynamic chemistry of Au3(pyrazolate)3 complexes. Just published in @daltontrans.rsc.org : pubs.rsc.org/en/Content/A...
#chemsky #EPFL
13.08.2025 10:35 —
👍 20
🔁 2
💬 1
📌 0

We have an opening in our institute at #EPFL for a tenure-track assistant professor position.
Please repost...
www.epfl.ch/about/workin...
22.07.2025 13:26 —
👍 27
🔁 22
💬 1
📌 0
Professeur-e ordinaire, professeur-e associé-e au Département de chimie physique (6453)
Full or associate professor at the Department of Physical Chemistry
At the University of Geneva, we have an opening for an associate or full professor in theoretical and/or computational chemistry! Please spread the word.
jobs.unige.ch/www/wd_porta...
18.07.2025 08:41 —
👍 2
🔁 4
💬 0
📌 0
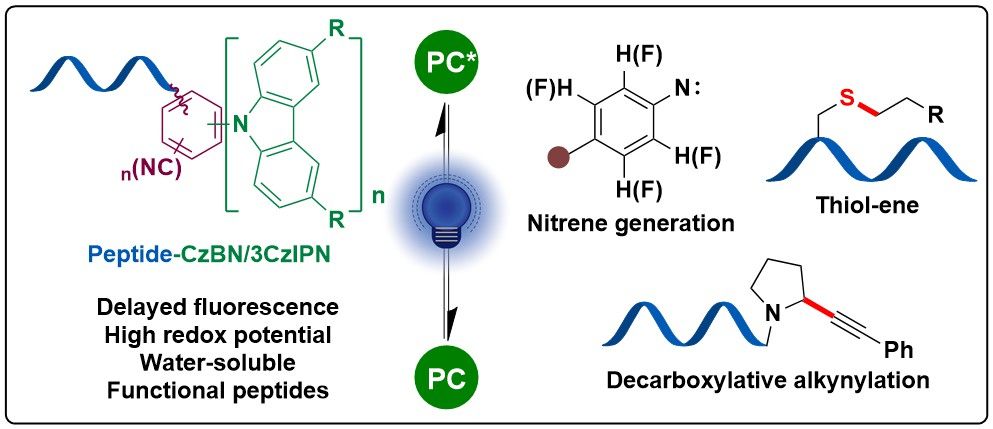
The work of Xingyu Liu @xingyuliu9595.bsky.social in collaboration with Wei Cai in Beat Fierz @beatfierz.bsky.social group @lcbm-epfl.bsky.social and Anne-Sophie Chauvin on peptide-cyanoarenes used as photocatalysts is now accepted in @angewandtechemie.bsky.social !
doi.org/10.1002/anie...
05.06.2025 18:57 —
👍 10
🔁 5
💬 1
📌 0

While many collaborations never culminate in a publication, others simply require time to mature. In this case, a full decade proved to be the necessary incubation period. Finally out in Chem. Eur. J.: chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1...
@irenebenzop.bsky.social #chemsky #EPFL
07.07.2025 06:32 —
👍 19
🔁 2
💬 0
📌 0
Unfortunately, Figure 1 and Scheme 1 were mixed up at the proof stage...
30.05.2025 12:25 —
👍 1
🔁 0
💬 0
📌 0
With help from Paul Varava (synthesis), Rosario Scopelliti (XRD), and Farzaneh Fadaei-Tirani (XRD). Thanks a lot!!
30.05.2025 12:25 —
👍 1
🔁 0
💬 1
📌 0

Dicky Wong (@thwongax.bsky.social ) has explored the chemistry of iridium complexes with N-heterocyclic vinylidene ligands. Just published in JACS (@jacs.acspublications.org ): pubs.acs.org/doi/10.1021/...
#chemsky #EPFL
30.05.2025 12:25 —
👍 34
🔁 3
💬 2
📌 1