 28.11.2024 14:49 — 👍 6 🔁 0 💬 0 📌 0
28.11.2024 14:49 — 👍 6 🔁 0 💬 0 📌 0
Xxi
@locuste.bsky.social
Scientific popularization, but not only. Posts in French and English.
@locuste.bsky.social
Scientific popularization, but not only. Posts in French and English.
 28.11.2024 14:49 — 👍 6 🔁 0 💬 0 📌 0
28.11.2024 14:49 — 👍 6 🔁 0 💬 0 📌 0
Bientôt, y en aura plus.
26.11.2024 20:34 — 👍 1 🔁 0 💬 0 📌 0Merci, on va essayer de toucher un public international, maintenant. 😅
Je compte postuler dans un feed prochainement.
Poke (How does snow form ?)
@drbarriere.bsky.social @lionelcase.bsky.social @corinnedepagne.bsky.social
Poke (How does snow form ?)
@chatsecouriste.bsky.social @saiyanbio.bsky.social @ericbilly.bsky.social @duxpacis.bsky.social @astropierre.com @lethiecv.bsky.social @ledoc.bsky.social @nopeneverhope.bsky.social @tipuncho.bsky.social @sonicurticant.bsky.social @mathieumolimard.bsky.social

That’s it, we’ve reached the end of this thread! If you enjoyed it, don’t forget to repost and like, and subscribe to stay updated with my future content. Thanks for your support and see you soon !
(Feel free to also follow my Threads account if you're interested : www.threads.net/@insightful_...)
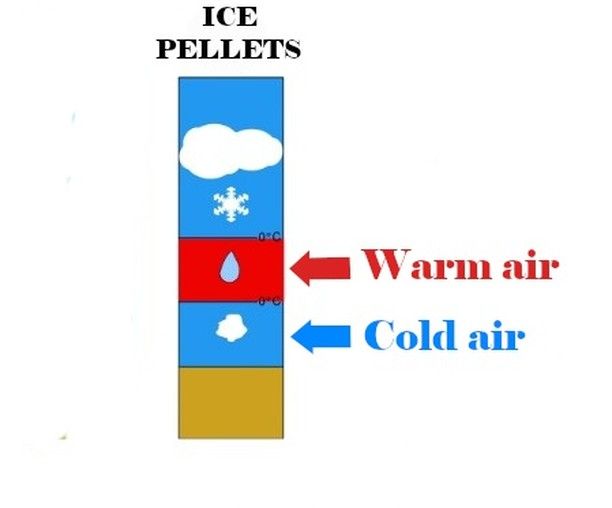

2- The air layer is sufficiently thickThis time, as you might have guessed, the droplets become solid again, taking the form of ice pellets.
26.11.2024 20:18 — 👍 1 🔁 0 💬 1 📌 0

This is the famous - and no less formidable - freezing rain !
26.11.2024 20:17 — 👍 0 🔁 0 💬 1 📌 0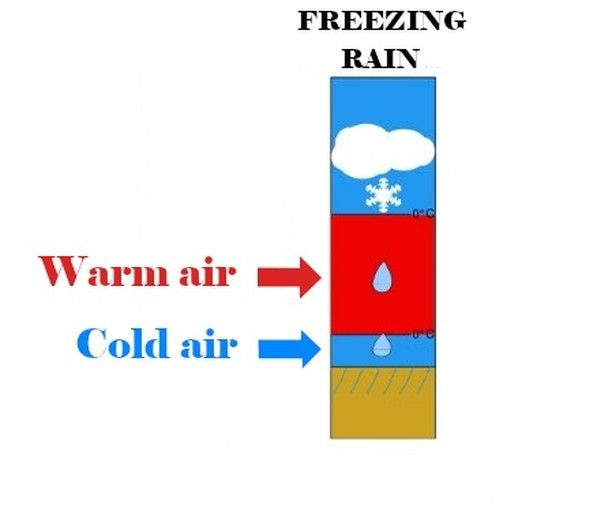
1- The air layer is too thin.Under these conditions, the droplets do not have enough time to return to the solid state; however, they become supercooled, which will eventually allow them to freeze upon contact with the ground (or other surfaces).
26.11.2024 20:16 — 👍 0 🔁 0 💬 1 📌 0But sometimes, they encounter a cold air layer again, just before landing!In this scenario, two outcomes are possible…
26.11.2024 20:16 — 👍 0 🔁 0 💬 1 📌 0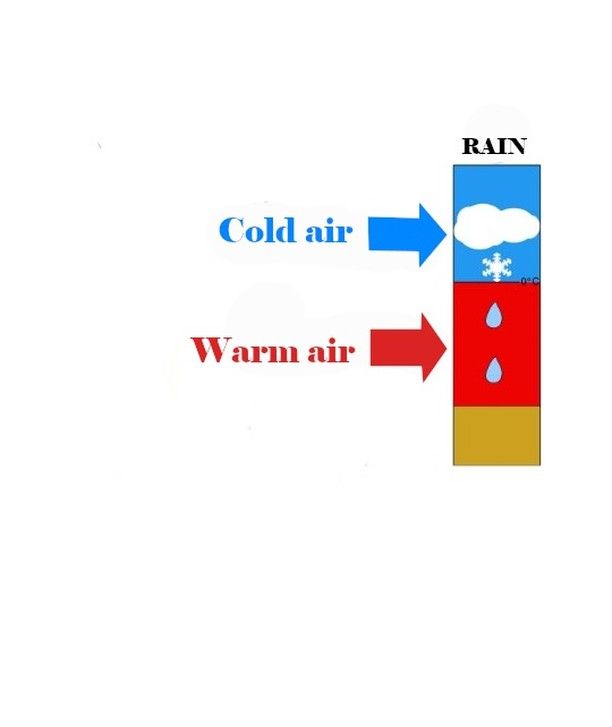
Of course, if they only pass through warm layers, the question doesn’t even arise: they will remain liquid until they reach the ground, and we will have to deal with regular rain…
26.11.2024 20:16 — 👍 0 🔁 0 💬 1 📌 0Even though there is no longer any hope for the snow to return at this stage, we still don’t know what will happen to the droplets that result from it, as they now have to pass through the remaining air layers before reaching us…
26.11.2024 20:16 — 👍 0 🔁 0 💬 1 📌 0
But if unfortunately, the snowflakes are exposed to warm temperatures (I mean temperatures significantly above 0°C), it’s a disaster: they melt!We then get ordinary raindrops…
26.11.2024 20:15 — 👍 0 🔁 0 💬 1 📌 0
They will then settle in successive layers on the ground, ultimately offering us a divine snowy blanket!
(It should be noted that the colder it is, the more likely we are to find dry snow. Conversely, temperatures closer to 0°C favor the appearance of wet or even slushy snow.)
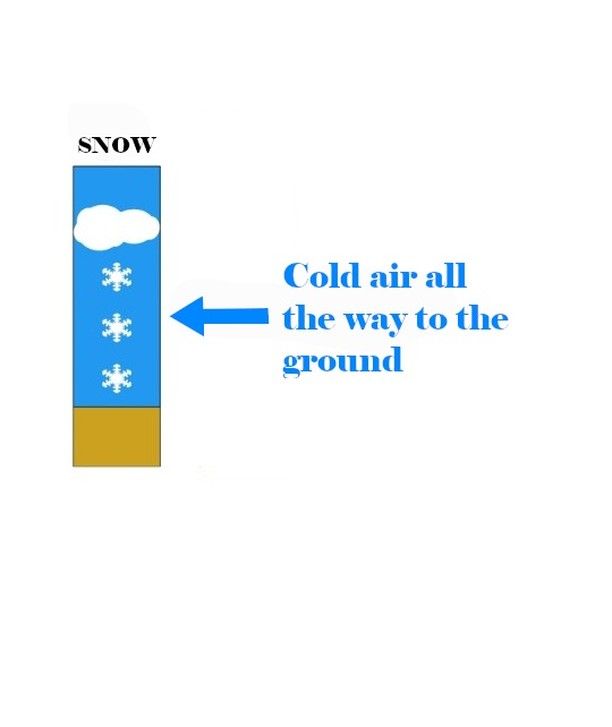
If all these layers are cold (in other words, if it is below 0°C, or, in the worst case, just a little above), the snowflakes will be preserved.
26.11.2024 20:14 — 👍 0 🔁 0 💬 1 📌 0Unfortunately, it should be noted that at this stage, there is still no guarantee that they will reach us in this form: everything will depend on the temperature of the air layers they have yet to cross…
26.11.2024 20:13 — 👍 0 🔁 0 💬 1 📌 0
More or less large, but always intertwined, these different clusters constitute what we call snowflakes!
Their fall is truly a magical spectacle…
That said, since they all share the common trait of being subjected to whimsical paths, collisions between them quickly multiply, which either breaks them apart or interlocks them with each other.It is therefore possible to see clusters of snow crystals forming all over the sky.
26.11.2024 20:11 — 👍 0 🔁 0 💬 1 📌 0
These random movements generate collisions with other snow crystals… At this moment, there are millions of them in the sky, each more beautiful than the other. All different too; as if each had its own identity. To be honest, I never get tired of admiring them…
26.11.2024 20:11 — 👍 0 🔁 0 💬 1 📌 0and falls to the ground…There, subjected to air turbulence, it does not descend in a straight line, but actually follows a capricious trajectory, punctuated by deviations and sudden jolts.
26.11.2024 20:10 — 👍 0 🔁 0 💬 1 📌 0I say almost, because don’t forget about the supercooled droplets! In case of a shock or even slight contact, which sometimes happens, they can also solidify on the crystal, increasing its volume.
If the crystal finds good quantities of them in its vicinity, it eventually becomes too heavy
it readily deposits on the protruding corners of the crystal, which are the most accessible).
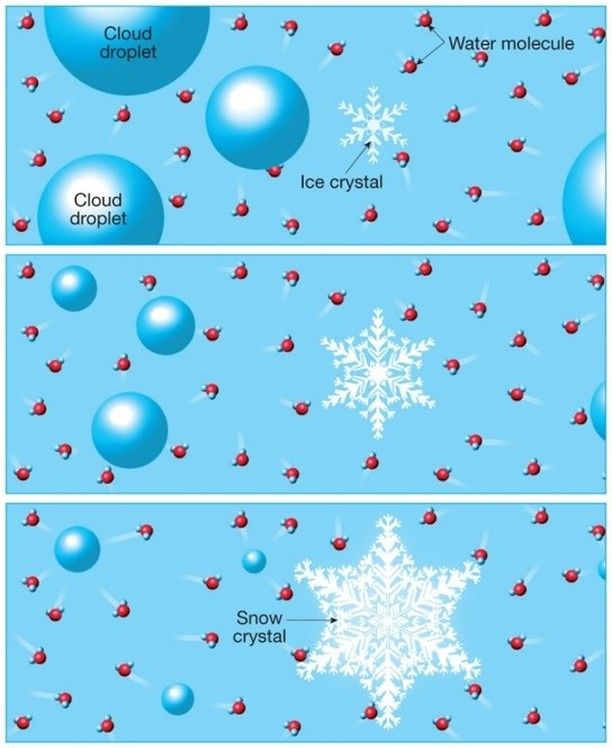
As you can see, water vapor plays a central role, not only in the formation but also in the development of the snow crystal. Almost everything depends on it.
It is triggered spontaneously due to the difference in humidity between the air around the crystal and that in the droplet zone.
Once in action, it induces the gradual evaporation of the droplets, and the water vapor thus formed joins the crystal, freezing onto it in turn (it should be noted that
So, if necessary, I prefer to warn you against the temptation to attribute any will to the snow crystal: no, I assure you, it is not consciously stealing the droplets to grow at their expense!
In reality, this transfer of water molecules is a natural process called the Bergeron effect.
their immediate transition to the solid state. We will see later examples of situations that cause this sudden change, but for now, let’s continue with my story.
As I mentioned, the snow crystal develops by inheriting water molecules that belonged to the neighboring droplets.
In such a state, water droplets can remain liquid at temperatures below 0°C, up to a limit that nature has set around -40°C.
However, since it is an unstable state, the slightest external disturbance (imagine contact with a surface or a particle, for example) will be enough to trigger
because we are in a cloud with negative temperatures. Shouldn’t the water freeze under these conditions ?
Well, actually, no ! Even below 0°C, water droplets will remain liquid.This paradox, which is troubling for our minds, actually corresponds to a particular state of matter called supercooling.
Although it starts as a tiny ice grain (and for good reason, it doesn’t exceed a few millimeters at most), it will have the opportunity to grow by drawing water molecules from the surrounding water droplets !
Now, I guess the persistence of these droplets might seem completely absurd to you,
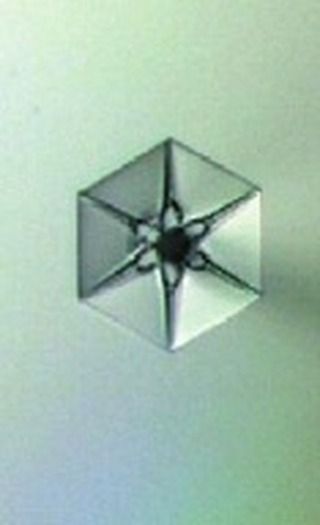
Therefore, it is not easy to encounter the right type of aerosol under the ideal climatic conditions!
But when things align well, we find ourselves in the presence of a fabulous primary snow crystal.
Credit : Kenneth Libbrecht
However, we have seen that not all aerosols are well-suited for this role. Worse, even among those that are capable, there are still inequalities.
Some only become active at very low temp (around -15°C, for example), while others, more accommodating, can perform their function starting at -3°C.