Thrilled to see our work published! Huge thanks to all my co-authors for the great collaboration!!
17.03.2025 12:50 — 👍 1 🔁 0 💬 0 📌 0
10/10 For a more detailed explanation, check out bsky.app/profile/thec... for an in-depth thread on our findings.
17.03.2025 12:49 — 👍 1 🔁 1 💬 0 📌 0
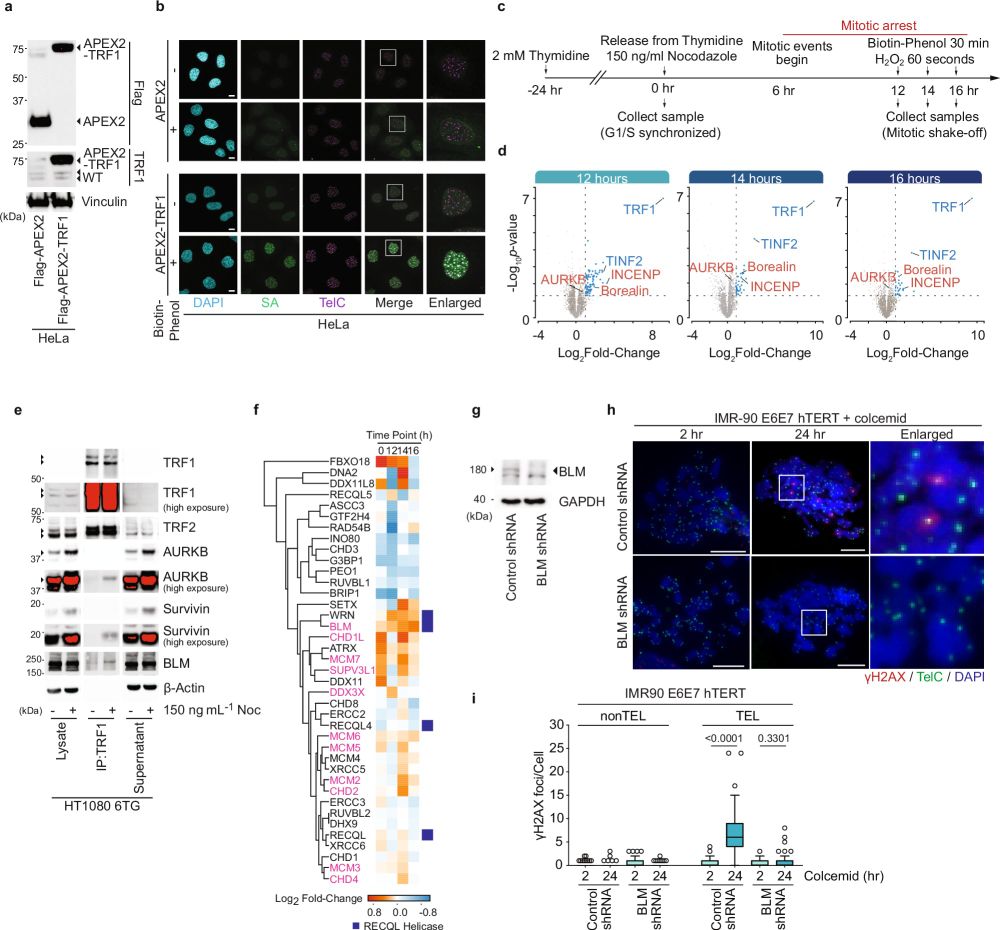
9/10 These insights not only advance our understanding of telomere biology but also opens potential therapeutic avenues for targeting mitotic death in cancer cells.
17.03.2025 12:49 — 👍 0 🔁 0 💬 1 📌 0
8/10 Our findings support a dynamic model where coordinated post-translational modifications of shelterin promote t-loop unwinding, aiding in the removal of damaged cells from the cell cycle.
17.03.2025 12:49 — 👍 0 🔁 0 💬 1 📌 0
7/10 This phosphorylation is essential for BTR-mediated double Holliday junction dissolution and contributes to mitotic telomere deprotection, highlighting a previously underappreciated role of the shelterin complex.
17.03.2025 12:49 — 👍 0 🔁 0 💬 1 📌 0
6/10 Shelterin is a telomere-binding protein complex that includes TRF1 and TRF2, crucial for maintaining telomere structure and function. During mitotic arrest, Aurora Kinase B (AURKB) of the CPC phosphorylates TRF1 and TRF2, leading to telomere linearity and a telomere-specific DDR.
17.03.2025 12:49 — 👍 0 🔁 0 💬 1 📌 0

5/10 Our study uncovers a sophisticated mechanism involving the Chromosome Passenger Complex (CPC), the shelterin complex, and the BLM-TOP3A-RMI1/2 (BTR) complex in regulating MAD-telomere deprotection.
17.03.2025 12:49 — 👍 0 🔁 0 💬 1 📌 0

4/10 Telomeres safeguard chromosome ends by forming t-loops, preventing ATM activation and unwarranted DNA damage responses (DDR). However, during mitotic arrest, telomere linearity and a localized DDR emerge—a phenomenon known as "Mitotic Arrest Dependent (MAD)-Telomere Deprotection."
17.03.2025 12:49 — 👍 0 🔁 0 💬 1 📌 0
3/10 Additional key contributors include Ronnie Low, Scott Page, Blake Lane, Andrew Robinson, and Lucy French from @thecesarelab.bsky.social. Fuyuki Ishikawa from Kyoto University and Shunya Kosaka from my lab.
17.03.2025 12:49 — 👍 0 🔁 0 💬 1 📌 0
2/10 This work highlights the power of collaboration. Special thanks to our fantastic collaborator @thecesarelab.bsky.social. Diana Romero from my lab and Sam Rogers from Cesare’s lab contributed equally to this work. Their perseverance truly deserves immense recognition!
17.03.2025 12:49 — 👍 0 🔁 0 💬 1 📌 0
Associate professor and CRUK Senior Fellow, University of Copenhagen and University of Oxford. My lab researches how DNA repair defects and loss of genome stability cause disease, including cancer.
https://icmm.ku.dk/english/research-groups/blackford-group
Scientist: durocherlab.org @ Lunenfeld-Tanenbaum Research Institute, Toronto.
Founder: Repare Therapeutics & Induxion Therapeutics
Head of Genome Stability Unit at SVI, Melbourne. All things DNA damage response: Fanconi Anaemia, Bloom Syndrome, Gene editing, R-loops, telomeres, HR & more
We study mechanisms promoting genome stability altered in cancer cells
Scientist. https://www.sfeirlab.com/
Prof. at MSKCC and co-founder of Repare Therapeutics. 🇱🇧+🇺🇸 in NYC
The Downs lab at the Institute of Cancer Research in London, studying chromatin biology and genome stability. thedownslab.org
Researcher, group leader, professor, mum of 2. Interested in DNA replication and damage, ubiquitylation, cell cycle, molecular and structural biochemistry.
Chasing DNA double strand break repair outcomes and clastogen response
https://scholar.google.com/citations?user=JZpgwJwAAAAJ&hl=en
Molecular biologist. Group leader at MRC LMB Cambridge UK. Fellow at Clare Hall college. #CryoEM #RNAbiology #DNArepair
Views/opinions are my own.
https://www2.mrc-lmb.cam.ac.uk/group-leaders/n-to-s/lori-passmore/
Professor | Gene Center @ LMU Munich
Cellular responses to DNA and RNA damage
We study chromosomal replication & recombinational repair mechanisms, Smc5/6, SUMO-based controls using multidisciplinary approaches. Tweets are a group effort.
The Fena Ochs Lab at the University of Copenhagen
3D chromatin function super-resolved
https://www.fenaochslab.org/
Genome Integrity Lab | Children's Research Institute, UT Southwestern Medical Center. We study how chromosomes segregate, break, and rearrange.
www.thelylab.org
Full professor at Leiden University Medical Center. Studying DNA repair in chromatin and its link to human disease.
www.vanattikumlab.org
Group leader at the University of Birmingham interested in rare diseases caused by inherited defects in DNA repair and replication. Amateur geologist/palaeontologist and shark enthusiast.
Studying DNA replication, repair and recombination using biochemical, biophysical and structural methods. University of Bristol, UK.
ORCID ID: 0000-0002-4612-7141
Group leader of the DDR lab at https://ipbs.fr Toulouse #CNRS. Science addict. Chemical biology and Genomics to find new druggable targets to treat #cancer & #aging. https://t.co/qZ65X6y1Qq
Genome Stability and Innate Immunity Lab at the Institute of Cancer Research, London
https://www.zierhutlab.org/
CNRS group leader at ENS de Lyon studying DNA recombination and spatial genome organization.
https://www.ens-lyon.fr/LBMC/equipes/mecanique-du-genome