💗The February 2026 issue of Trends in Chemistry is out now!
Our cover highlights the Review from Sarah Park and colleagues on chiral reticular frameworks as emerging spin filters.
Read all the articles in our latest issue here: www.cell.com/trends/chemi...
11.02.2026 17:54 — 👍 1 🔁 0 💬 1 📌 1

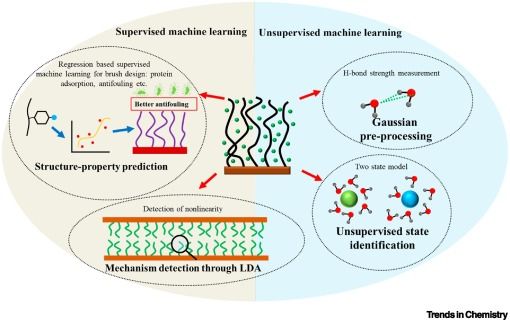
Molecularly imprinted functional monomers: from tradition to customization
Molecularly imprinted polymer (MIPs) possess exceptional molecular recognition capabilities, making them highly valuable for applications in separation, sensing, environmental monitoring, and biomedicine. Functional monomers play a central role in determining MIP performance; yet MIPs prepared using traditional functional monomers often suffer from poor selectivity and limited capabilities, which severely restricts the efficacy of MIPs in highly complex systems. Recent advances in computational chemistry, machine learning, and biomimetic strategies have enabled the rational design of customized monomers with improved affinity, responsiveness, and environmental compatibility. Despite these developments, the field lacks a systematic overview of the paradigm shift in functional monomers design – particularly how interdisciplinary integration can overcome the limitations of conventional approaches – and a coherent research framework remains undefined. This review systematically traces the development of functional monomers, emphasizing key structure–function relationships and design principles. It explores how customized monomers drive innovation in fields like trace contaminant detection, chiral separation, and smart drug delivery. Continued interdisciplinary advances are expected to usher MIPs into a new era of intelligent, sustainable design.
Online now: Molecularly imprinted functional monomers: from tradition to customization
20.01.2026 13:42 — 👍 0 🔁 0 💬 0 📌 0
Artists choice - it was clearly a very cool cover!
15.01.2026 19:47 — 👍 1 🔁 0 💬 0 📌 0

📆The January 2026 issue of Trends in Chemistry is online now!
www.cell.com/trends/chemi...
The cover highlights the Opinion article from Bruce Lipshutz, looking at Pd vs Ni cross-coupling reactions in terms of synthetic efficiency and sustainability.
Cover art credit: Peter Allen, Second Bay Studios
14.01.2026 17:35 — 👍 2 🔁 1 💬 0 📌 1
Divergent heterocycle synthesis enabled by switchable reaction of azobenzenes with alkynes
Azobenzenes are iconic photochromic molecules whose reversible light-induced E–Z isomerization underpins decades of applications in dyes, polymers, molecular switches, and therapeutic agents. Beyond this hallmark, their potential as synthetic building blocks has only recently emerged. In particular, the reactivity of azobenzenes with alkynes is unveiling a new frontier in heterocycle synthesis. Under metal-catalyzed, photocatalytic, or thermal conditions, these transformations provide streamlined access to indazoles, cinnolines, carbazoles, and related nitrogen heterocycles relevant to pharmaceuticals and materials science. This review discusses how distinct activation modes, transition-metal catalysis, photoredox processes, and thermal pathways enable divergent outcomes from common precursors, repositioning azobenzenes from passive chromophores to active scaffolds for functional molecular architectures.
Online now: Divergent heterocycle synthesis enabled by switchable reaction of azobenzenes with alkynes
06.01.2026 13:42 — 👍 1 🔁 0 💬 0 📌 0

Chiral reticular frameworks as emerging spin filters
Chiral induced spin selectivity (CISS) is a phenomenon wherein chiral materials preferentially transmit electrons of one spin over the other. Although interest in CISS is high and growing, its implementation in reticular frameworks, such as metal–organic frameworks (MOFs) and covalent organic frameworks (COFs), remains nascent. This review highlights advances in chiral reticular frameworks exhibiting CISS, emphasizing their intrinsic advantages rooted in modularity, tunability, and crystallinity. We examine reported examples across MOFs, COFs, and related crystalline systems, identify features correlated with high spin selectivity, and propose tentative design principles. By surveying this emerging intersection of reticular chemistry and chirality-induced spin selectivity, we aim to stimulate further exploration of chiral frameworks as next-generation spin filters.
Online now: Chiral reticular frameworks as emerging spin filters
16.12.2025 13:42 — 👍 0 🔁 0 💬 0 📌 0

🕊️🧬A Special Issue of Trends in Chemistry in online now!
www.cell.com/trends/chemi...
Our Dec 2025 issue is joint with @cp-cellrepphyssci.bsky.social on The Chemistry of Nucleic Acids, highlighting the innovations and thought leadership on nucleic acid research in the physical/chemical sciences.
15.12.2025 18:24 — 👍 5 🔁 6 💬 0 📌 0
Redirecting
Happy to share our review article @cp-trendschem.bsky.social on modified aptamers. Congrats to all authors!
@pasteur.fr @ipdbsc.bsky.social @cnrs.fr
doi.org/10.1016/j.tr...
06.12.2025 11:17 — 👍 9 🔁 3 💬 0 📌 0

[3+n]-Syntheses of carbocycles and heterocycles
During the past decade, metallovinyl carbenes derived from vinyldiazo compounds have emerged as facile dipolar sources of three-carbon synthons for cycloaddition reactions. Unlike traditional dipolar species that are limited to [3+2]-cycloaddition, metallovinyl carbenes are capable of [3+n]-cycloaddition, where transformations with n = 1, 2, and 3 are now abundantly demonstrated. The vinyldiazo compounds, the reacting n-dipoles, and the catalysts all play a role in determining the effectiveness of the cycloaddition process, with siloxyvinyldiazo compounds being optimum, the n-dipoles being highly variable, and dirhodium(II) and copper(I) catalysts providing the highest yields and selectivities among those surveyed. This brief opinion article provides basic strategies for [3+n]-cycloaddition transformations of vinyldiazo compounds and outlooks for their future developments involving carbene chemistry with versatile precursors.
Online now: [3+n]-Syntheses of carbocycles and heterocycles
08.12.2025 20:48 — 👍 0 🔁 0 💬 0 📌 0
Palladium versus Earth-abundant metals: comparisons from the synthetic and environmental perspectives for Suzuki–Miyaura cross-couplings and aminations
Direct comparisons are made using factual, published experimental results for two of the most commonly used cross-couplings that rely on either Pd or Ni (and in some cases, Cu, Co, and Fe) catalysis. Which metal in ligated form is best from both the synthetic and environmental perspectives for the intended bond formation is assessed comparing representative recent results for both Suzuki–Miyaura (SM) couplings and aminations. Several factors are considered, including: catalyst loadings, overall reaction efficiency, ligand availability, cost, residual metal expected in the products, energy invested, etc., and how these relate to the presumed benefits noted as the basis for shifting towards catalysis away from Pd using Earth-abundant metals (EAMs). For each type of coupling, a chart summarizing comparison data is provided, with the overall outcome leading to the same conclusion.
Online now: Palladium versus Earth-abundant metals: comparisons from the synthetic and environmental perspectives for Suzuki–Miyaura cross-couplings and aminations
04.12.2025 13:42 — 👍 1 🔁 0 💬 0 📌 1

Jess is heading to Boston next week for MRS Fall 2025 🧪
Get in touch if you want to share your ideas about the latest trends in materials chemistry; it's also a great opportunity to find out more about the chemistry/materials publishing opportunities at Cell Press! #F25MRS
24.11.2025 16:15 — 👍 3 🔁 2 💬 0 📌 0
Redirecting
Our mini-review on DNA-scaffolded catalysis is out today in @cp-trendschem.bsky.social! It's an interesting look at this unique intersection of catalysis, DNA nanotechnology and supramolecular chemistry. Give it a read! #chemsky doi.org/10.1016/j.tr...
09.11.2025 05:26 — 👍 3 🔁 1 💬 1 📌 0

⚡The latest issue of Trends in Chemistry is online now!
The cover shines a spotlight on two articles in this month's issue covering electrochemistry for complex molecule synthesis.
Read the full issue: www.cell.com/trends/chemi...
13.11.2025 14:19 — 👍 0 🔁 0 💬 0 📌 0
Assistant Professor, UW-Madison Chemistry. Group website: http://martellgroup.chem.wisc.edu.
Assistant Professor at Bar-Ilan University. Department of Chemistry
💊 PharmD • PhD Pharmacology • University of Houston
🦠 BS Biology • University of Texas at Austin
🌐 JacobKMcPherson.com (business)
🔬 McPhersonLab.github.io (academic)
Chem Circularity is the newest Cell Press journal for cutting-edge insights and research to advance the pursuit of closed-loop solutions.
International Research Training Group 2991 on "Photoluminescence in Supramolecular Matrices" - IISER Thiruvananthapuram 🇮🇳 and JMU Würzburg 🇩🇪
Website: https://www.uni-wuerzburg.de/irtg2991
Self-assembly, supramolecular structures, functional properties and device applications of organic dye materials @uni-wuerzburg.de. Account managed by group members.
https://www.chemie.uni-wuerzburg.de/oc/wuerthner-group/
Using quantitative, data-driven approaches to make synthetic chemistry more predictable. Phase II CCI #NSFFunded
🌐 https://ccas.nd.edu/
📷 https://www.instagram.com/nsf_ccas/
Lecturer in Chemistry @ University of Aberdeen 🏴 | Liquid Crystals💧💎 | Cat Mom to Raven and Raja 🐾
Peptide self assembly, evolution, and bioinorganic chemistry. Assistant professor at University of Illinois Chicago (UIC)
ainguyenlab.com
Developing cutting-edge analytical platforms for comprehensive studies of biological systems. Specializing in ambient ionization mass spectrometry profiling, imaging, and real-time volatile organic compounds (VOCs) detection.
Postdoc@ETH Zürich Polymer Chemistry Group
PhD@SNU Chem
The Sofer Research Group located, led by Prof Zdeněk Sofer. We are located at the University of Chemistry and Technology Prague (VŠCHT Praha/ UCT Prague), Czechia. Research on 2D layered materials and wide range of applications.
https://sofergroup.cz/
Group Leader @TLClab @ETH_Materials, Interested in Polymer Science, Organic Chemistry and Material Science. Alumni @Caltech @MerkleInstitute @unifr
Incoming Assistant Professor at Northwestern University. Current postdoc in Nocera Lab at @ Harvard; Former: PhD @ Johns Hopkins (Goldberg Lab); UG @ UF (Murray Lab); he/him
Computational chemist @ Department of Chemistry "Giacomo Ciamician", University of Bologna, Italy
We are a research group at UC San Diego led by Professor Andrew Pun in the Department of Chemistry & Biochemistry.
Student-run AP Lab account.
Supramolecular Chemist @cnrs.fr @unistra.fr
Acridinium to Viridium Chemistry
Multi-responsive Components
The Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg, Germany is the first institution of its kind worldwide to combine ribonucleic acid (RNA) research with infection biology.
https://www.helmholtz-hiri.de/en/imprint/
Organic chemistry research group at Newcastle University @chemistryncl.bsky.social. Formerly at the FU Berlin.