
Big day for my group yesterday when my first graduate student, Daniela Carmona-Pérez, passed her candidacy exam with flying colors! 🎉 Stay tuned for her next paper! (coming soon)
20.05.2025 17:30 — 👍 11 🔁 0 💬 0 📌 0@aet-ice.bsky.social
Assistant Professor in Chemistry at University of Rochester; PI of Thorarinsdottir Group; PD Harvard; PhD Northwestern University; Inorganic chemist who likes electrochemistry Group website: https://sas.rochester.edu/chm/groups/thorarinsdottir/

Big day for my group yesterday when my first graduate student, Daniela Carmona-Pérez, passed her candidacy exam with flying colors! 🎉 Stay tuned for her next paper! (coming soon)
20.05.2025 17:30 — 👍 11 🔁 0 💬 0 📌 0
Check out our Viewpoint article in ACS Energy Letters on variable-temperature electrochemistry🔌⚡🌡️ pubs.acs.org/doi/10.1021/...
07.03.2025 15:39 — 👍 5 🔁 1 💬 0 📌 0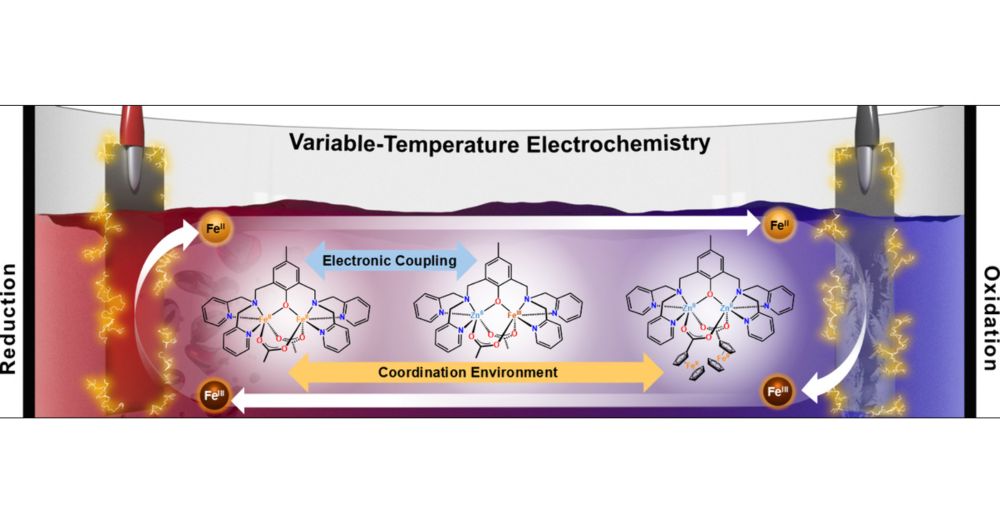
I am thrilled to share this paper from my group that is just out in ACS Electrochemistry. We study factors that impact the temperature sensitivity of redox potentials in iron complexes. You can read about it here (it is open access): pubs.acs.org/doi/10.1021/...
18.02.2025 11:22 — 👍 21 🔁 2 💬 0 📌 0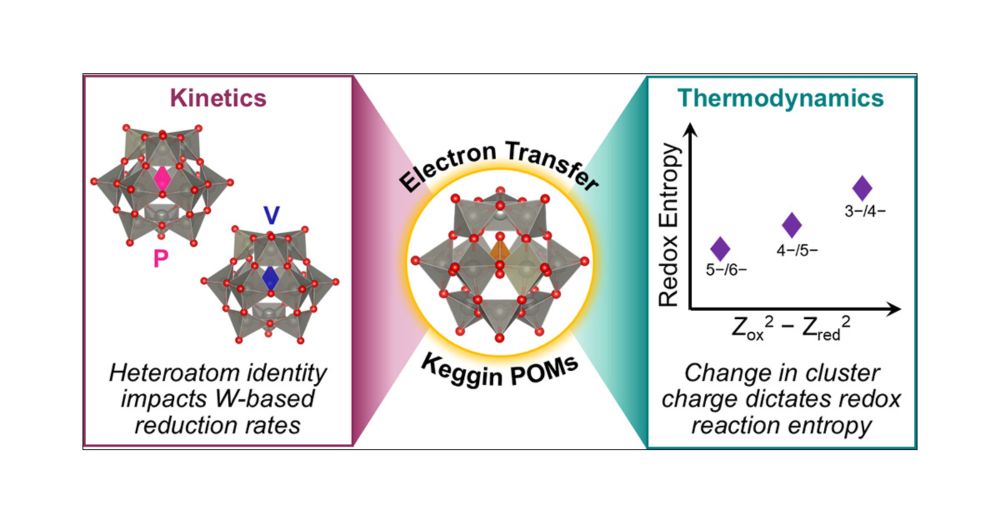
We had a blast collaborating with @matsonlab.bsky.social Check out our paper in ACS Materials Au pubs.acs.org/doi/10.1021/... Special shout out to the amazing Mamta Dagar!!!
28.11.2024 13:07 — 👍 12 🔁 1 💬 0 📌 1