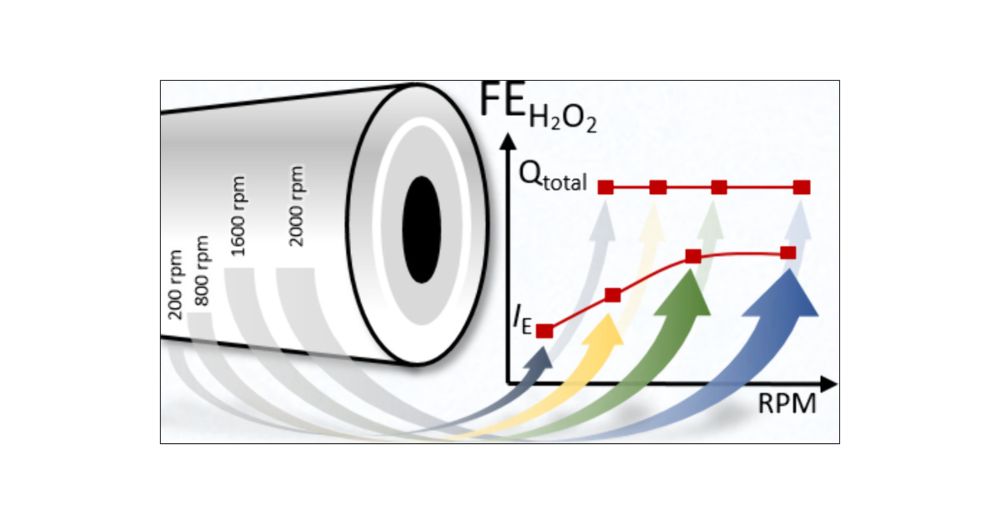
2. Apparent lower FEs at low rotation rates can be easily caused by experimental conditions. These effects need be explicitly eliminated in order to observe possible influences of mass transport or species' retention times in RRDE experiments at different rotation rates.
27.08.2025 09:52 — 👍 0 🔁 0 💬 0 📌 0
1. FE can be accurately determined even at low rotation rates, but this requires certain precautions: experimentally determining the collection efficiency under the relevant conditions, observing the total charge associated with the ring current due to current peak delay and widening, or both
27.08.2025 09:52 — 👍 0 🔁 0 💬 0 📌 0
I am an electrochemist with 10+ years experience in academic research:
scholar.google.com/citations?us...
20.05.2025 20:48 — 👍 1 🔁 0 💬 1 📌 0
LinkedIn
This link will take you to a page that’s not on LinkedIn
Check out our new paper in the International Journal of Hydrogen Energy: www.sciencedirect.com/science/arti...
We explore the effects of catalyst dispersion and ink formation on electroanalytic performance in RDE and MEA configurations.
Cooperation: @unidue.bsky.social, ZBT, and @maxplanck.de
14.05.2025 17:19 — 👍 1 🔁 0 💬 0 📌 0
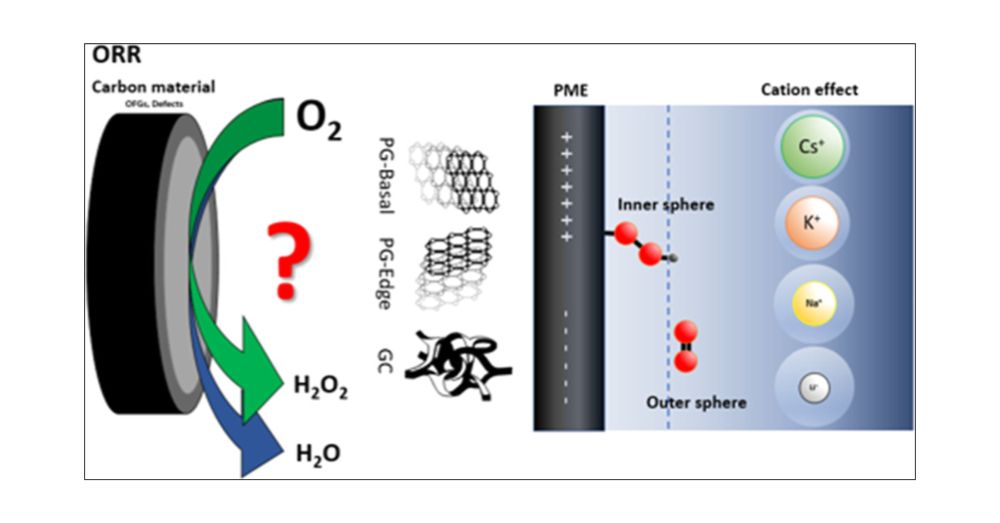
Electrochemical Insights into Hydrogen Peroxide Generation on Carbon Electrodes: Influence of Defects, Oxygen Functional Groups, and Alkali Metals in the Electrolyte
Hydrogen peroxide (H2O2) is an environmentally friendly oxidant, with production reaching 5.7 million tons by 2028 and a market size of USD 4.04 billion by 2029. Understanding the mechanism of oxygen reduction to H2O2 and the structure–activity relations on carbon materials is, therefore, of high significance for the more environmentally friendly synthesis of this important chemical. We have used oriented pyrolytic graphite (PG-edge and PG-basal) and glassy carbon (GC) as model electrodes to investigate the influence of carbon defects, oxygen-containing functional groups, and the presence of alkali metals on the activity and selectivity toward H2O2 production under acidic conditions. Electrochemical measurements, such as rotating ring disk electrode and electrochemical impedance spectroscopy, as well as in situ Raman spectroelectrochemistry indicated that PG-basal and GC electrodes preferentially form H2O2 as the product through the two-electron pathway via inner and outer sphere mechanisms, respectively. The mechanism is significantly affected by the potential of maximal entropy, which determines the position of species in the solution within the inner or outer Helmholtz plane. The influence of alkali cations (Li+, Na+, K+, and Cs+) on the oxygen reduction reaction of these model carbon electrodes was investigated. Large cations, e.g., K+ and Cs+, showed influence on the reaction intermediates and thus on the electrodes’ selectivity. The present study provides important insights and contributions to the fundamental aspects of hydrogen peroxide production in acidic conditions and further advancements in the development of metal-free carbon-based catalysts.
Our new paper in ACS Catalysis discusses ORR on oriented graphitic carbon materials, their fundamental electrochemical properties and surface-structure-dependent differences in reaction mechanisms. A long and complex story, with hopefully more to come!
pubs.acs.org/doi/10.1021/...
09.12.2024 14:23 — 👍 3 🔁 1 💬 0 📌 0
A charitable foundation supporting the communication and dissemination of information in #chemistry and related disciplines.
We support #OpenScience projects – #DiamondOA publishing, standards, events, cheminformatics
https://www.beilstein-institut.de/en
🇺🇦Ukrainian blogger.. 🪖Military analytics and weapons.Geopolitics.. Monitoring of global conflict zones ||🇺🇦~🇷🇺||🇮🇱~🇮🇷||🇹🇼~🇨🇳||🇵🇰~🇮🇳||Ukraine will win
⭐️ Astronomer – MSc, PhD, Professor (교수)
⭐️ Seoul National University / 서울대학교
⭐️ Black holes, AGN, radio astronomy, VLBI
⭐️ Born at 332 ppm CO₂
⭐️ Trigger warning: math, physics, irony
⭐️ Signature: (+,-,-,-)
Hier postet das Ressort Presse
Links: http://uni-due.online/links
Impressum: udue.de/impressum
Datenschutz: udue.de/datenschutzerklaerung
Netiquette: udue.de/netiquette
Dr of Zoology
Data Scientist
Science feed co-creator
NYT bestselling author of #DoesItFart🐢💨
https://www.unimedizin-mainz.de/medizinische-mikrobiologie-und-hygiene/
#DGfI #DGHM #EFIS #IUIS / 🦖☄️🦠🔬🧫🐁🧍/ #immunometabolism / #host-pathogen interaction / 🇪🇺 / #UM #JGU: excellence in basic research and health care / own views only
Die DFG ist die größte Forschungsförderorganisation und die Selbstverwaltungsorganisation der Wissenschaft in Deutschland.
www.dfg.de
Impressum: https://www.dfg.de/de/service/kontakt/impressum
Social Media-Netiquette: https://www.dfg.de/de/service/presse
News from the German Foreign Office - Follow us in German as well @diplo.de
Imprint and data privacy: diplo.de/2697714
Assistant professor at Chemistry 🥼⚗️🧪, University of Copenhagen 🇩🇰, excited about electrocatalysis and X-rays 💫
Co-Founder and CEO at iNSyT Solutions - University of Munich (LMU), Germany
Microscopy 🔬 Electrochemistry ⚡️
LinkedIn (iNSyT Solutions): https://www.linkedin.com/company/insyt-tech?trk=profile-position
From 🇵🇱, based in Cologne 🇩🇪
PhD in Physics (🇵🇱)
Postdoc in Physical Chemistry (🇩🇪)
past: Research Coordinator @RWTH Aachen & MPI CEC
Lindau-Alumna
Programme Officer @DFG (🇩🇪)
At the interface of electroanalysis and materials, using small electrodes, imaging, and the power of our diversity to solve energy challenges. Opinions are our own, 1st person is PI Joaquin. Learn about us https://rodriguezlopez.chemistry.illinois.edu
Professor of Electrochemistry at Department of Materials, Imperial College London. Leader of Interfacial Electrochemistry Group. Henry Royce Institute: Research Area Lead in Atoms to Devices.
Venezuelan 🇻🇪 in Germany 🇩🇪. Mom/Amateur Baker/Electrochemist/Editor-in-Chief of ChemElectroChem and Batteries & Supercaps. Views my own.
🇪🇺 energy wonk | sci&eng’ing the shit out of eChem NH₃ synthesis www.NitroVolt.com 🇩🇰 | bits of past in 🇩🇪🇭🇰🇮🇹🇺🇸 | born@350ppm
Professor at DTU Physics, Head of section, ERC CoG
Electrochemistry for Energy Conversion research group is a part of the Max-Planck-Institute for Chemical Energy Conversion.
Research group leader: https://bsky.app/profile/viktorcolic.bsky.social