
Want to read about some of the highlights in the broad field of coordination chemistry from 2025? Look no further!
gdch.app/article/nebe...
Put together by myself and (Bluesky-absent) Gabi Hierlmeier 🧪
@coburgerpeter.bsky.social
www.coburger-lab.de We are a research group @ TUM, focussing on the reactivity of biradicaloid complexes.

Want to read about some of the highlights in the broad field of coordination chemistry from 2025? Look no further!
gdch.app/article/nebe...
Put together by myself and (Bluesky-absent) Gabi Hierlmeier 🧪

@townrowresearch.bsky.social and I summarized some recent developments in molecular main-group chemistry. A fun project with many beautiful new discoveries last year! 👨🔬👩🔬
The annual "Trendbericht" can i.e. be read here:
gdch.app/article/haup...
Just 4 weeks left to apply for the PhD position in our research group! Deadline: 31 January. Don’t miss out, apply today :)
05.01.2026 10:05 — 👍 5 🔁 9 💬 0 📌 0
Thrilled to share that Soti's Dalton Trans. paper on Ta(V) PCET chemistry ranked among the most popular articles of 2025! 🥳 Huge thanks to everyone who read and supported our work 🙌
www.linkedin.com/pulse/dalton...
Paper: pubs.rsc.org/en/content/a...
All the best for your next station 😊
29.12.2025 13:18 — 👍 1 🔁 0 💬 1 📌 0Happy to see this fun project with @kretschmerlab.bsky.social out.
Two gallium centers in close proximity led to some unexpected outcomes.
Thanks to everyone involved and congrats to the team!

@scheschkewitz.bsky.social and I shine a light on how main group element motifs - incl. multiple bonds - impact the photophysical properties of conjugated polymers and pave the way for new applications 🎨🔦 Check out our review @angewandtechemie.bsky.social
onlinelibrary.wiley.com/doi/10.1002/...

Nucleophilic #Bismuth: diarylbismuth-anions grant access to bismuth-acyl compounds & visible-light-induced reversible insertion/extrusion of CO! Just out @natcomms.nature.com: link.springer.com/article/10.1.... Congrats to the team (including @felixgeist.bsky.social, @jordipoater.bsky.social)!
20.11.2025 19:52 — 👍 34 🔁 12 💬 5 📌 1
Unusual coordination chemistry & reactivity: the methyl #bismuth dication shows a pentagonal pyramidal coordination and can be turned into a Lewis #superacid. Read more about Johannes', @taminazk.bsky.social, and Bene's work in @angewandtechemie.bsky.social: onlinelibrary.wiley.com/doi/10.1002/...!
17.11.2025 11:36 — 👍 27 🔁 4 💬 2 📌 1
What happens if you combine phosgene, neutron diffraction and DFT? A Tale of polymorphism, and intermolecular interactions! Three years in the making and finally published in @angewandtechemie.bsky.social: Congrats, Sven! #molecules #phosgene #neutrons doi.org/10.1002/ange...
14.11.2025 19:40 — 👍 8 🔁 1 💬 1 📌 0
We are advertising for two PhD studentships in organometallic chemistry! Come and work with us @imperialchemistry.bsky.social from October 2026:
Advert 2) www.findaphd.com/phds/project...

We are advertising for two PhD studentships in organometallic chemistry! Come and work with us @imperialchemistry.bsky.social from October 2026:
Advert 1) www.findaphd.com/phds/project...

🚨 PhD Opportunity 🚨 🔁 Reposts appreciated!
My group at @manchester.ac.uk is recruiting a 3.5-year PhD student to explore Sb & Bi pincer complexes for metallomimetic chemistry.
🔬 Fully funded (UK only)
📍 Manchester
🧪 Synthetic inorganic chemistry
Apply 👉 tinyurl.com/yrjkvha9
Excited to share our new preprint! 🥳
We developed a modular strategy to access small four-membered biradicaloids by trapping them in Mn and Co complexes. Many congratulations to Stefan and Michi for their successful efforts !

How to catalytically cyclize alkyl species onto alkynes? Just add a pinch of bismuth! Read about our findings in controlled radical reactions catalyzed by well-defined manganese- bismuth heterobimetallic complexes, just published in ACS Catalysis @pubs.acs.org: pubs.acs.org/doi/10.1021/...
13.08.2025 15:27 — 👍 27 🔁 7 💬 2 📌 1
We present PhD No.4 from the group: Congrats Dr. Ayu! A splendid performance and a PhD period filled with laughter, lab curiosities and brilliant chemistry. Thanks Ayu and all the best for your future!
Also thanks @pchem.bsky.social for being on the committee. #phdone #proudpi #31p
Well deserved, even got the VIP tack 😅
14.10.2025 17:29 — 👍 0 🔁 0 💬 1 📌 0
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202515545#
Our group's very first ACIE paper is out!🥳
Awesome work by Soti and a great cooperation with the Bittl group and @franemmerling.bsky.social.
Dedicated to @christianlimberg.bsky.social and Franc Meyer
@humboldtuni.bsky.social @manchester.ac.uk
onlinelibrary.wiley.com/doi/full/10....

After some time, Annika's work on the first open-shell metallo-tetrylene is out 🥳 And it got the @chemicalscience.rsc.org
#PickoftheWeek stamp of approval, congrats Anni!🎖️
Great collab with Max Holthausen, (Frankfurt) 🖥️
Best thing here is the mechanism, so check it out:
tinyurl.com/4u9e5mvn


Soti and Sina presented their latest results at the NDDK this week. After just two months in the lab, Sina secured her first conference prize! Huge congratulations to her 🥳! We had a wonderful time, topped off by a buffet and drink selection of unparalleled quality and abundance.
27.09.2025 16:08 — 👍 11 🔁 1 💬 2 📌 0Well deserved , congratulations 🥳
16.09.2025 08:44 — 👍 1 🔁 0 💬 0 📌 0Much looking forward to be, more closely than ever, working with the European & global inorganic chemistry communities!
More musings on #EurJIC, its legacy and my biggest thanks to my predecessor, Preeti Vashi, in my inaugural editorial: chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/... 🧪

Another lovely talk from a @tum.de colleague @coburgerpeter.bsky.social here in Namur at the @mgerc-unamur.bsky.social ⚡🎉⚡
09.09.2025 14:05 — 👍 15 🔁 3 💬 0 📌 01 week left to apply for a PhD position, 4 y. with 2/3 of E 13 TV-L salary, assigned broadly to the design and synthesis of coordination compds for the activation of small molecules (www.chemie.hu-berlin.de/aglimberg/st...). Pls apply directly to christian.limberg@chemie.hu-berlin.de. Pls retweet!
14.08.2025 06:50 — 👍 10 🔁 13 💬 0 📌 1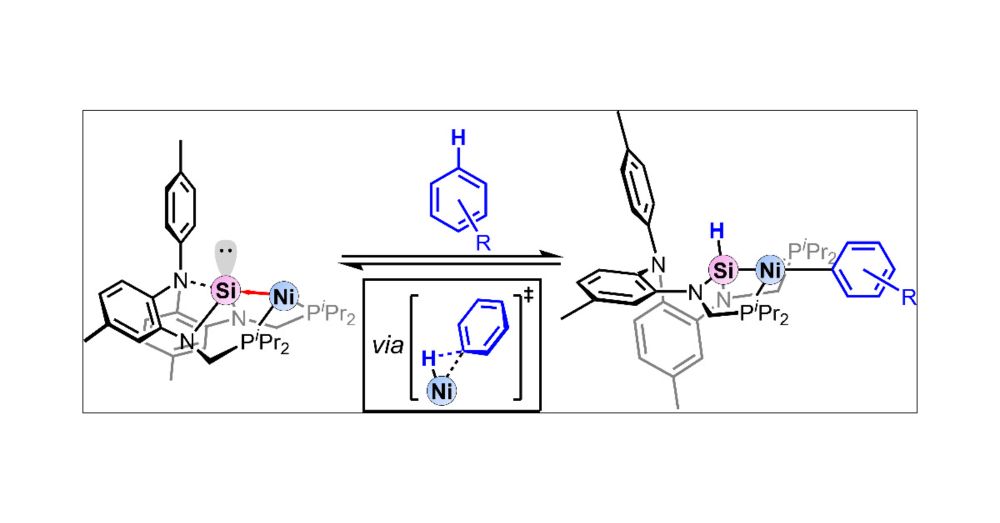
Reversible C–H Bond Activation of Unactivated Arenes by a Nickel-Silylene Complex - pubs.acs.org/doi/10.1021/...
So proud to congratulate first author Leon Gomm for his first paper in JACS. Thanks to Hui Zhu and Stefan Grimme for the ever helpful DFT calculations. @unibonn.bsky.social
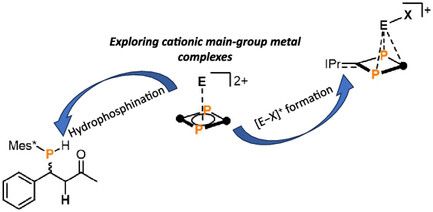
Our latest publication reports the successful isolation of π-stabilized tetryliumylidenes [E–X]+ starting from pyramidanes based on a biradicaloid ligand . This milestone was made possible thanks to David’s relentless efforts . Proud of the team!
26.07.2025 13:35 — 👍 15 🔁 2 💬 1 📌 0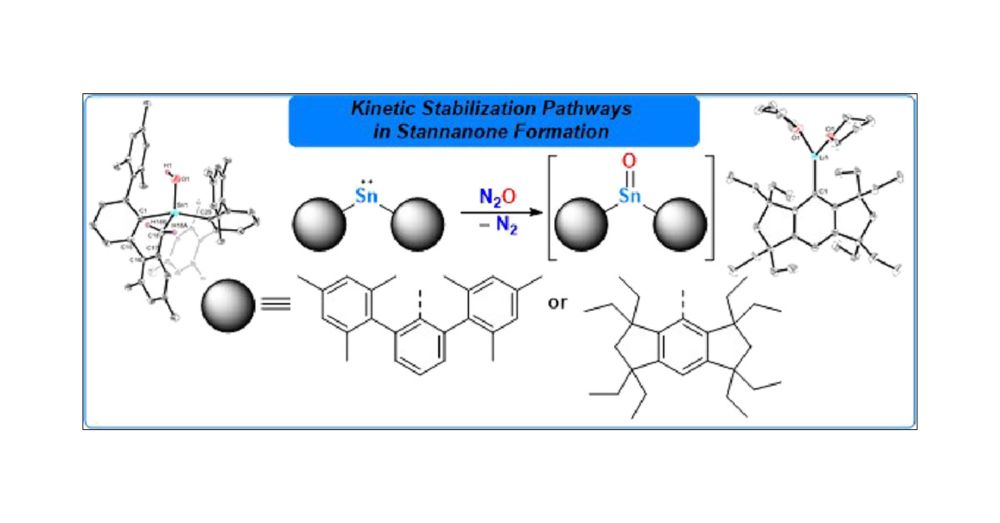
Excited to share our contribution to the special issue "Organometallic Chemistry Beyond the Transition Metals: Fundamentals and Applications of the P-Block" – now published @Organometallics! Check it out 👉 @pubs.acs.org #ChemSky
pubs.acs.org/doi/10.1021/...

Our review has been awarded the front cover of Inorganic Chemistry 🥰 @acs.org Thank you Stephan @hohlochlab.bsky.social for the great collab!
pubs.acs.org/cms/10.1021/...

We are very happy to announce that Prof. Müller has been awarded an honorary doctorate by the Budapest University of Technology and Economics. The collaborations with our friends from Hungary are always very fruitful and interesting!
03.06.2025 07:51 — 👍 11 🔁 2 💬 1 📌 0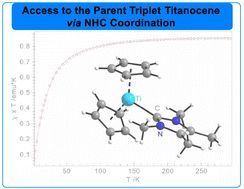
🔓Explore recent #OpenAccess work by @maltefischer.bsky.social, @townrowresearch.bsky.social and colleagues on the isolation of the parent triplet titanocene via NHC stabilisation🔥
pubs.rsc.org/en/content/a...
📍@unigoettingen.bsky.social and @kit.edu
🧪