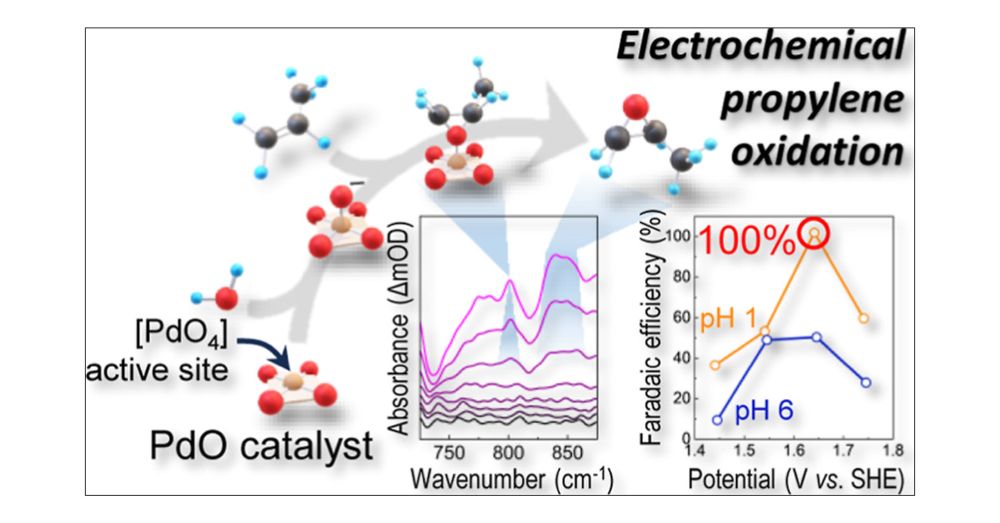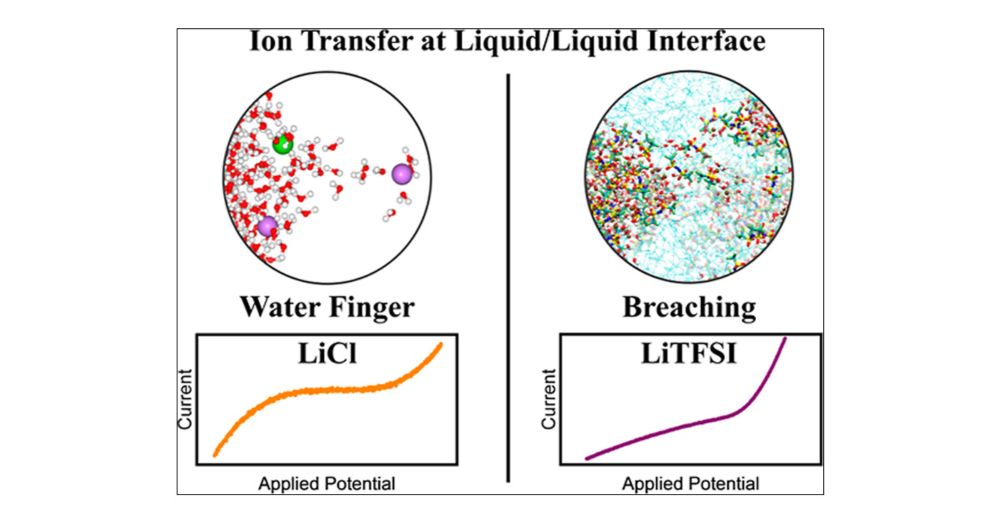
Polarization-Induced Breaching of the Liquid/Liquid Interface Formed with Water-in-Salt Electrolytes
The solvation properties of water-in-salt electrolytes (WiSEs) have been extensively studied by spectroscopic and computational means and were shown to impart them with unique chemical and physical properties when compared to more classical superconcentrated aqueous solutions. More specifically, the formation of ionic aggregates in solutions containing a large concentration of TFSI anions was shown to alter the water and anion reactivity at electrochemical interfaces, often improving the performance of aqueous rechargeable batteries. However, insights into the role of the WiSE solvation structure on ion transfer at electrochemical interfaces are scarce. Herein, interfaces between two immiscible electrolytes (ITIESs) are used to study the energetics for ion transfer between aqueous LiCl and LiTFSI solutions and dichloroethane. Combining electrochemical measurements at microinterfaces with metadynamics molecular dynamics (MD) simulations, the effect of solvation properties on the energy for transferring Li+ and Cl–/TFSI– ions across the liquid/liquid interface was studied. While increasing the LiCl concentration increases the amount of ion pairs, it only marginally impacts the ion transfer energy. Instead, using large LiTFSI concentrations at which ionic aggregates are formed, ion transfer across the liquid/liquid interface shows a unique behavior that departs from that observed for polarizable or nonpolarizable interfaces. Ions do not freely cross the interface, with a transfer energy found to be ≈8–10 kcal/mol. However, upon polarization, ionic aggregates are found to breach the liquid/liquid interface, locally mixing both solutions. We believe that such a finding calls for reevaluating our current understanding of ion transfer across chemical interfaces in superconcentrated electrolytes, including liquid/liquid interfaces used in membrane-less electrochemical systems.
Excited to see this work coming out @jacs.acspublications.org. Congrats Lihao on showing the unique transfer mechanism of ionic aggregates forming in Water-in-salt electrolytes across a liquid/liquid interface, with breaching of the interface upon polarization
pubs.acs.org/doi/10.1021/...
29.05.2025 20:34 — 👍 1 🔁 0 💬 0 📌 0
Flagship journal for @rsc.org, open access with no publication fee, all topics in chemistry. chemicalscience-rsc@rsc.org
Celebrating our 15th year in 2025! 🌐 Website: rsc.li/chemscience
Research, news, and information from the Journal of the American Chemical Society: pubs.acs.org/JACS
Clean energy advocate, materials scientist, battery researcher and professor
Journaliste. Red' chef adjoint magazine La Recherche (@maglarecherche.bsky.social). Je parle (livres de) science et intérêts perso : 📰 🏀 🚲 📷 🐦 🇨🇴 🇮🇪... Chercheuses et chercheurs, mes MP sont ouverts si vous avez une idée d'article à nous proposer.
A chemistry journal for cutting-edge research and analysis from @natureportfolio.nature.com. Account run by @nchemgav.bsky.social and @joansp.bsky.social
https://www.nature.com/nchem/
ICREA Professor at @icn2.bsky.social
Head of @nanoesc.bsky.social
ERC CoG @atomistic-erc.bsky.social
Académica en @academiajoven.bsky.social
https://www.nanoesclab.com/
Assistant Professor 🔋⚡️🤖🧪, Department of Chemistry - Ångström Laboratory, Uppsala University, Sweden. Interested in battery interphases, operando characterizations, robotics, and mechanics. https://leitingzhang.com
Professor at EPFL (Ecole Polytechnic Fédérale de Lausanne). Http://lsci.epfl.ch; catalysis - heterogeneous, homogeneous, bio; membrane; electrochemical energy device. Co-founder and chairman, NovaMea. Http://novamea.swiss
Chemistry professor at Missouri S&T, electrochemist, editor-in-chief of the ACS Au journals
Chemistry research group at Johns Hopkins University interested in inorganic chemistry and materials discovery for energy storage and conversion.
Research laboratory at Cambridge University. Our work focuses on artificial photosynthesis to make renewable fuels.☀️☀️PhD-run account.
Virtual Chemist; Liúdramán; Personal Opinions
Assistant professor at Chemistry 🥼⚗️🧪, University of Copenhagen 🇩🇰, excited about electrocatalysis and X-rays 💫
Rob Armstrong's Battery Research Lab at University of St Andrews
World's leading facility in neutron science & tech for +50 years with
our associate countries 🇫🇷 🇩🇪 🇬🇧
and scientific member countries 🇦🇹 🇨🇿 🇩🇰 🇮🇹 🇵🇱 🇸🇰 🇪🇸 🇸🇪 🇸🇮 🇨🇭
We are based in Grenoble, FRANCE
Senior Lecturer in the Chemistry Department at Imperial College London. Working on🔋
Dad of 2, Prof. @ the University of Houston and National University of Singapore. Energy storage is the game 🔋🪫
A new @natureportfolio.bsky.social journal publishing leading research, analysis, and commentary covering the breadth and depth of chemical engineering.
https://www.nature.com/natchemeng/
Updates on the McCrory Lab at the University of Michigan. https://sites.lsa.umich.edu/mccrory-lab
Professor of Electrochemistry at Department of Materials, Imperial College London. Leader of Interfacial Electrochemistry Group. Henry Royce Institute: Research Area Lead in Atoms to Devices.