
ICYMI: ONLINE NOW: Microvascular hypoxia and inflammation in chronic pain syndromes
10.12.2025 02:13 — 👍 1 🔁 0 💬 0 📌 0@cp-trendsmolecmed.bsky.social
Reviews journal by Cell Press publishes articles covering all aspects of human diseases, diagnostics, therapeutics, and disease prevention. Posts are by the editor, Dr. Aliki Perdikari. Explore more at: https://www.cell.com/trends/molecular-medicine/home

ICYMI: ONLINE NOW: Microvascular hypoxia and inflammation in chronic pain syndromes
10.12.2025 02:13 — 👍 1 🔁 0 💬 0 📌 0
ONLINE NOW: Cholesterol metabolism: a new checkpoint in cancer immunity
10.12.2025 00:38 — 👍 1 🔁 0 💬 0 📌 0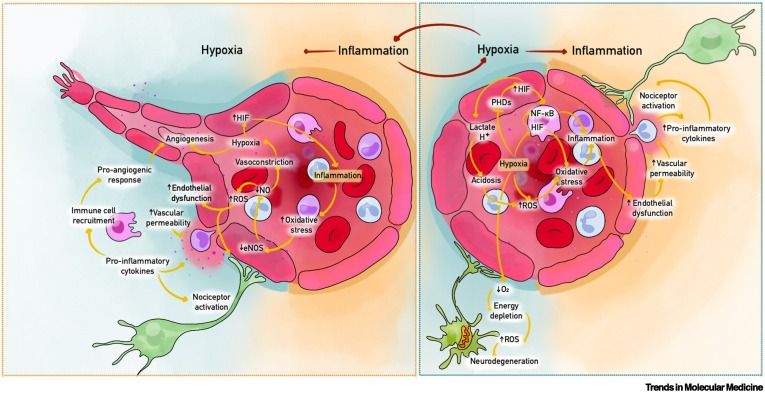
ONLINE NOW: Microvascular hypoxia and inflammation in chronic pain syndromes
08.12.2025 20:12 — 👍 0 🔁 0 💬 0 📌 0
ICYMI: ONLINE NOW: The overlooked uterine factor: unlocking endometrium potential in premature ovarian insufficiency
07.12.2025 18:53 — 👍 0 🔁 0 💬 0 📌 0
ONLINE NOW: The overlooked uterine factor: unlocking endometrium potential in premature ovarian insufficiency
06.12.2025 12:52 — 👍 1 🔁 0 💬 0 📌 0
ICYMI: ONLINE NOW: Repurposing of microbial proteins as new-generation therapeutic agents for biomedical applications
03.12.2025 06:39 — 👍 0 🔁 0 💬 0 📌 0
ICYMI: ONLINE NOW: Neuroendocrine timekeepers: changes in normal and premature aging
02.12.2025 18:55 — 👍 1 🔁 0 💬 0 📌 0
ONLINE NOW: Repurposing of microbial proteins as new-generation therapeutic agents for biomedical applications
02.12.2025 00:37 — 👍 0 🔁 0 💬 0 📌 0
ONLINE NOW: Neuroendocrine timekeepers: changes in normal and premature aging
01.12.2025 12:53 — 👍 0 🔁 0 💬 0 📌 0
ICYMI: ONLINE NOW: Predicting healthspan and disease risks through biological age
29.11.2025 18:54 — 👍 0 🔁 0 💬 0 📌 0
ICYMI: ONLINE NOW: The right drug at the right time: key to IBD success
29.11.2025 02:13 — 👍 2 🔁 0 💬 0 📌 0
ONLINE NOW: Predicting healthspan and disease risks through biological age
28.11.2025 12:52 — 👍 2 🔁 1 💬 0 📌 0
ICYMI: ONLINE NOW: Tryptophan metabolites at the service of neuroimmune sensing of microbes
21.11.2025 02:13 — 👍 1 🔁 0 💬 0 📌 0
ICYMI: ONLINE NOW: Emerging innovative treatments for leptomeningeal metastatic tumors
20.11.2025 06:38 — 👍 2 🔁 0 💬 0 📌 0
ONLINE NOW: Tryptophan metabolites at the service of neuroimmune sensing of microbes
19.11.2025 20:12 — 👍 2 🔁 0 💬 0 📌 0
ONLINE NOW: Emerging innovative treatments for leptomeningeal metastatic tumors
19.11.2025 00:37 — 👍 0 🔁 0 💬 0 📌 0
ICYMI: ONLINE NOW: Molecular advances and therapeutic potential in leveraging hepcidin
14.11.2025 06:40 — 👍 1 🔁 0 💬 0 📌 0
ICYMI: ONLINE NOW: Androgen receptor and its coregulators in sex-biased diseases
14.11.2025 02:13 — 👍 1 🔁 0 💬 0 📌 0
ONLINE NOW: Molecular advances and therapeutic potential in leveraging hepcidin
13.11.2025 00:38 — 👍 2 🔁 0 💬 0 📌 0
ONLINE NOW: Androgen receptor and its coregulators in sex-biased diseases
12.11.2025 20:12 — 👍 0 🔁 0 💬 0 📌 0
The November issue of Trends in Molecular Medicine is now online! www.cell.com/trends/molec...
Special thanks to all the authors and reviewers for their contribution!
I am happy to receive your feedback and ideas for topics so please get in touch!

ICYMI: ONLINE NOW: Transcriptional landscape of skeletal muscle in cancer patients
01.11.2025 17:53 — 👍 1 🔁 0 💬 0 📌 0
ONLINE NOW: Transcriptional landscape of skeletal muscle in cancer patients
31.10.2025 11:52 — 👍 0 🔁 0 💬 0 📌 0
ICYMI: ONLINE NOW: ANGPTL3/8: one target, multiple lipid disorders
30.10.2025 17:57 — 👍 0 🔁 0 💬 0 📌 0ICYMI: ONLINE NOW: Precision medicine for sodium channelopathy-related autism and epilepsy
29.10.2025 20:06 — 👍 0 🔁 0 💬 0 📌 0ONLINE NOW: Precision medicine for sodium channelopathy-related autism and epilepsy
28.10.2025 14:04 — 👍 0 🔁 0 💬 0 📌 0