
Excited to share this short video about our lab's work! Thank you Harvard Medicine News Team! @harvardmicro.bsky.social @hhmi-science.bsky.social @harvard.edu
❤️🎥😃https://www.youtube.com/shorts/GFM3KLZCDps

Excited to share this short video about our lab's work! Thank you Harvard Medicine News Team! @harvardmicro.bsky.social @hhmi-science.bsky.social @harvard.edu
❤️🎥😃https://www.youtube.com/shorts/GFM3KLZCDps
Thank you, Blair!!!
30.12.2025 18:32 — 👍 1 🔁 0 💬 0 📌 0Thank you to everyone involved, including @joeloparo.bsky.social @kranzuschlab.bsky.social !
29.12.2025 20:34 — 👍 0 🔁 0 💬 0 📌 0HPIs provide a promising new approach to combatting herpesvirus drug resistance. Our study elucidates their inhibition mechanisms and offers insight into herpesvirus replisome assembly, providing a basis for further inhibitor development 💊
29.12.2025 20:34 — 👍 0 🔁 0 💬 1 📌 0
We also show that helicase residue K356 plays a key role in stabilizing HPI binding to the HSV-1 H/P complex. UL52 residues used in drug binding are poorly conserved across β- and γ-herpesviruses, a possible explanation for α-herpesvirus drug specificity.
29.12.2025 20:34 — 👍 0 🔁 0 💬 1 📌 0Using optical tweezers experiments with @JoeLoparo lab, we show that HPIs block DNA translocation such that the complex pauses on DNA. Structural analysis reveals that HPIs lock 🔒the UL5 helicase in an open conformation, preventing motor domains from closing.
29.12.2025 20:34 — 👍 0 🔁 0 💬 1 📌 0
We discovered an extensive interface between polymerase (UL30) and helicase (UL5), mediated in part by a conserved motif within the polymerase.
29.12.2025 20:34 — 👍 0 🔁 0 💬 1 📌 0
Cryo-EM maps of H/P subunits UL5, UL52, and UL8 reveal a bilobed architecture centered on primase UL52. Further addition of the polymerase holoenzyme UL30 and UL42 allowed us to understand how the HSV-1 replication fork assembles onto DNA and its inhibition by HPI drugs.
29.12.2025 20:34 — 👍 0 🔁 0 💬 1 📌 0The HSV helicase-primase (H/P) complex–involved in viral genome replication–is a promising target for emerging drugs, known as helicase-primase inhibitors (HPIs) 💊 Our recent @CellCellPress paper uses cryo-EM to reveal mechanisms behind why these new drugs are effective.
29.12.2025 20:34 — 👍 1 🔁 0 💬 1 📌 0Herpesviruses are prevalent pathogens, with HSV-1 causing orolabial infections and severe disease in immunocompromised patients 😷Because of the risk HSV-1 developing resistance to current drugs, new antiviral strategies are a priority.
29.12.2025 20:34 — 👍 0 🔁 0 💬 1 📌 0
We are excited to share our new @CellCellPress paper revealing mechanisms of action of promising drugs against the helicase-primase of herpes simplex virus 1 (HSV-1) 🤩 harvardvirology.bsky.social www.cell.com/cell/fulltex...
29.12.2025 20:34 — 👍 7 🔁 3 💬 1 📌 1
Now online! Mechanisms of HSV-1 helicase-primase inhibition and replication fork complex assembly
29.12.2025 19:52 — 👍 3 🔁 4 💬 0 📌 0
#NewResearch
Cryo-EM structures of the full-length Junin virus and Machupo virus spike glycoprotein complexes stabilized in the prefusion conformation - analyses reveal features that regulate glycoprotein pH-dependent membrane fusion activity.
#MicroSky 🦠
www.nature.com/articles/s41...
Though the Candid#1 vaccine is effective against JUNV, other lethal New World arenaviruses still pose a large public health risk 🌎with no specific medical countermeasures. The structures may serve as a basis for further rational design and development of therapeutics and vaccines
09.08.2025 09:51 — 👍 1 🔁 0 💬 0 📌 0
A K33A substitution in the stable signal peptide (SSP) allowed for stabilization of the pre-fusion GPC, with molecular dynamics suggesting K33A stabilizes GPC by better accommodating membrane lipids in the SSP33 pocket, supported by our cryo-EM density
09.08.2025 09:51 — 👍 0 🔁 0 💬 1 📌 0
Further structural and functional analyses also reveal the effect of specific residues in transmembrane and membrane proximal region on pH-mediated fusion, elucidating the attenuation mechanism of the JUNV Candid#1 vaccine
09.08.2025 09:51 — 👍 0 🔁 0 💬 1 📌 0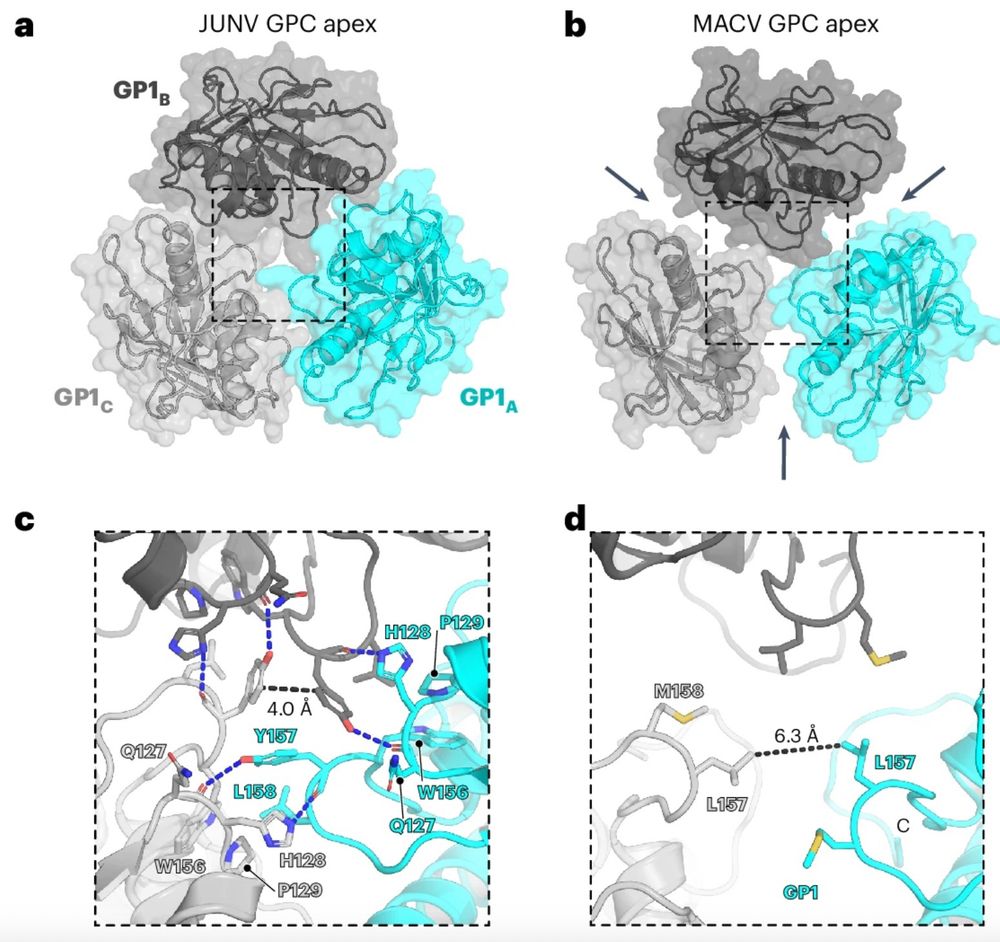
These cryo-EM structures reveal notable differences between JUNV and MACV in GP1 organization at the apex and C terminus. The MACV GPC apex has a more open appearance due to its GP1 subunits slightly rotating outwards and clockwise 🔃compared to JUNV GP1 subunits
09.08.2025 09:51 — 👍 0 🔁 0 💬 1 📌 0
Our @natmicrobiol.nature.com paper provides the most complete insight into the molecular organization of the JUNV and MACV GPCs yet 😲revealing new features not seen in previous structures, such as the TM helices and cytosolic portions, including ZBD
09.08.2025 09:51 — 👍 0 🔁 0 💬 1 📌 0New World arenaviruses, like Junin virus (JUNV) and Machupo virus (MACV), cause hemorrhagic fever with fatality rates as high as 30% predominantly in South America. To date, the only medical countermeasure against these viruses is the Candid#1 vaccine effective only against JUNV
09.08.2025 09:51 — 👍 0 🔁 0 💬 1 📌 0
Excited to share our new @natmicrobiol.nature.com paper revealing the most complete arenavirus glycoprotein complex (GPC) structures to date 🤩 www.nature.com/articles/s41...
09.08.2025 09:51 — 👍 17 🔁 12 💬 1 📌 1
Now online! Molecular basis for shifted receptor recognition by an encephalitic arbovirus
05.04.2025 12:42 — 👍 7 🔁 3 💬 0 📌 0
Also sharing the terrific news piece by Jake Miller at HMS News on our latest paper! @cp-cell.bsky.social
hms.harvard.edu/news/how-sma...
WEEV re-emerged in South America in 2023-2024 in a large outbreak, highlighting the critical need for preparedness. Using the structures, we identified E2 polymorphisms that predict WEEV strain receptor dependency, which will support environmental surveillance.
04.04.2025 19:58 — 👍 3 🔁 0 💬 0 📌 0
Interesting tidbit in the story: a WEEV-related alphavirus that circulates on the east coast (EEEV territory) called highlands J virus (HJV) also uses PCDH10 as a receptor. It diverged from WEEV not that long ago and retained most PCDH10 contact residues.
04.04.2025 19:58 — 👍 5 🔁 1 💬 1 📌 0
Some WEEV strains and EEEV both bind VLDLR at the LA1 and LA2 domains, suggesting that VLDLR LA1-LA2 decoy receptor may have broad activity against them. This molecule protected mice against lethal challenge by a WEEV strain that binds VLDLR.
04.04.2025 19:58 — 👍 1 🔁 0 💬 1 📌 0
Strains isolated during epidemics of the early 20th century bind VLDLR and ApoER2 as additional receptors. Our structure of a 1941 strain bound by VLDLR revealed a distinct binding mode than other alphaviruses that bind LDLR-related proteins, such as EEEV, SFV or VEEV.
04.04.2025 19:58 — 👍 0 🔁 0 💬 1 📌 0
We found a substitution in the E2 glycoprotein, L149Q, that would disrupt hydrophobic contact with PCDH10, and confirmed this mutation alone abolishes human PCDH10 binding. L149Q is found in all strains isolated in California and Texas in 2005, suggesting a wide geographic range.
04.04.2025 19:58 — 👍 1 🔁 0 💬 1 📌 0
Protocadherin 10 (PCDH10) is a major receptor for WEEV. Multiple strains isolated in 2005 lost the ability to bind human and horse PCDH10, yet still bind avian PCDH10. Cryo-EM structures show identical binding mode of sparrow and human PCDH10.
04.04.2025 19:58 — 👍 1 🔁 0 💬 1 📌 0WEEV is a mosquito-borne alphavirus that caused widespread outbreaks in horses and humans in the early 20th century, but outbreak frequency and scale dramatically decreased since then. We previously found this “submergence” of WEEV was accompanied by shifted receptor recognition.🦟🐎
04.04.2025 19:58 — 👍 1 🔁 0 💬 1 📌 0
Our study on the molecular basis of shifts in receptor recognition by western equine encephalitis virus (WEEV) in the past century, led by Xiaoyi Fan and @wanyuviridae.bsky.social , is online! 👏🎉
www.cell.com/cell/fulltex...