Check out our new work synthesizing (P^N^C)Gold(III) complexes, creating two new Au-C bonds in one pot! @chem.uzh.ch
07.09.2025 16:32 — 👍 3 🔁 0 💬 0 📌 0

Promotional graphic featuring the table of contents image along with the journal and article title.
'Synthesis of (P^N^C)Gold(III) Complexes via Tandem Oxidative Addition/C–H Auration' from ACS Organic & Inorganic Au is an open access #ACSEditorsChoice.
📖 Read the article: buff.ly/2UUBEnb
07.09.2025 12:01 — 👍 2 🔁 1 💬 0 📌 1


Fantastic two weeks of science! Very happy to have participated at the Bienal @rseq-quimica.bsky.social and @eucomc2025.bsky.social sharing our advances in gold(III) chemistry, made at the @chem-uzh-ch.bsky.social. Thanks to the organisers for these great opportunities! @swisschemistry.bsky.social
11.07.2025 15:58 — 👍 10 🔁 3 💬 2 📌 0

Interrogating the anti-Insertion of Alkynes into Gold(III)
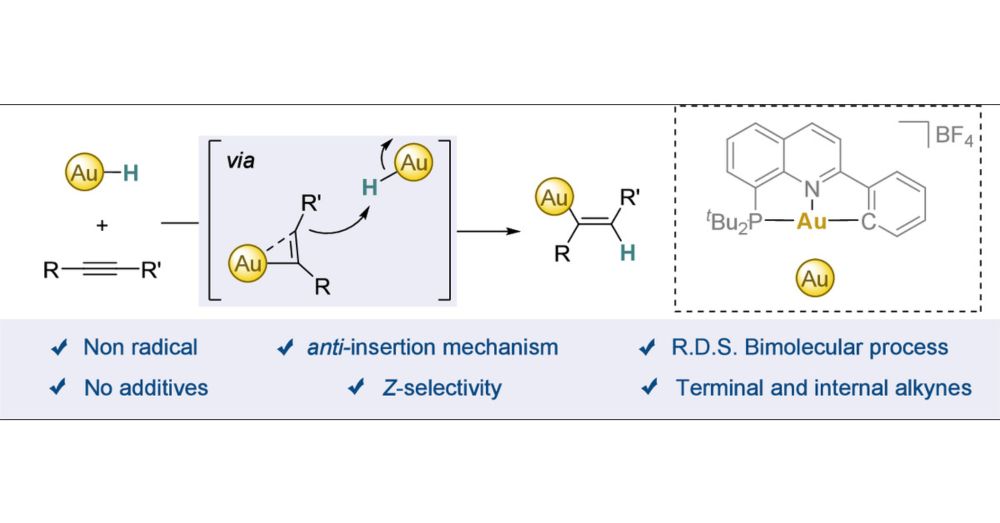
Alkyne hydrofunctionalizations are a powerful strategy to efficiently build up structural complexity. The selectivity of these reactions is typically governed by the interaction between the alkyne and a metal-hydride, which commonly proceeds via a well-understood syn-insertion mechanism. In contrast, anti-insertions are far less common, with proposed mechanisms often extrapolated from literature precedents rather than grounded in direct experimental evidence. While gold complexes rank among the most efficient catalysts for such transformations, the mechanistic understanding of the key alkyne insertion step remains incomplete. In this study, we demonstrate that stable gold(III)-hydrides, featuring a (P∧N∧C) ligand, undergo selective insertion of alkynes to yield the corresponding anti-Markovnikov Z-vinyl complexes. A combination of control experiments, kinetic studies, and computational analyses reveals a nonradical, bimolecular insertion process, in which water plays a pivotal role by accelerating the reaction and potentially stabilizing a highly reactive, T-shaped gold(I) intermediate. Notably, this is the first demonstration of the insertion of both activated and unactivated terminal and internal alkynes into a gold(III)-hydride complex.
My first post here comes with my latest work at the University of Zurich: Interrogating the anti-Insertion of Alkynes into Gold(III)! We explore the complex mechanism behind this apparently simple two-component reaction. Check it out in JACS Au pubs.acs.org/doi/10.1021/...
@uzhchemistry.bsky.social
28.02.2025 07:58 — 👍 3 🔁 1 💬 0 📌 0
Inorganic Chemistry Professor at Alcala University. SOSCATCHEM Group leader. Organometallic Chemistry, Main Group metals, Catalysis, Polymers, Bioplastics, Halogen Bonding.
https://soscatcom.es/
Mother, Wife, Chemistry Professor, CRC, Head of Department, Start Up Founder. At UBC-Vancouver, we develop new early transition metals catalysts with specialization in amine molecules and materials.
Passionate about homogeneous catalysis, ligand design, and organometallic chemistry; can’t spend enough time in the mountains
Professor of chemistry at the EPFL, Switzerland
lcs.epfl.ch
Assistant Professor (W1) at @Uni_MR. Organometallic chemistry and sustainable catalysis. | mum | Views are my own
Senior editor @naturechemistry.bsky.social handling coordination chemistry. Before: @NatureComms, PhD @IQCCUdG, MSCA fellow @WardgroupBS | Opinions are my own
XXVI European Conference on Organometallic Chemistry
July 6-10, 2025, University of Bern, Switzerland #EUCOMC2025
https://eucomc2025.scg.ch
La RSEQ es una sociedad, declarada de utilidad pública, que tiene por objeto promover, desarrollar y divulgar la disciplina de la Química, tanto en su aspecto de ciencia pura como en el de sus aplicaciones, en todo el ámbito del estado español.
Marie Curie Postdoctoral Fellow at Holland Group. Yale University.
Self-assembled materials
Postdoc in the Wennemers Group, ETHZ
PhD in the Rickhaus Group, UZH
MSCA postdoctoral fellow at IIQ-CSIC (Seville, Spain). Organometallic chemistry, Main group chemistry, Catalysis.
ACS Publications is proud to publish the most trusted, most cited, and most read collection of journals in the chemical and related sciences.
Explore ACS Publications, a division of @acs.org: pubs.acs.org
Tenured Scientist at ISQCH (CSIC-UZ)
Tenured Scientist @ ISQCH (CSIC-UZ) Bioinorganic Chemistry Research Lab: Development of Photosensitisers, Chemotherapeutic and Theragnostic agents. https://sites.google.com/view/pdt-section-vfm-group
Organometallic chemist with a writer's soul. Associate Professor at UAM. She/her
Professor of Chemistry at University of Bern , Synthesis, Catalysis, s-block metals, mum of two, prod European, runner #chemistry #maingroupchemistry #catalysis #organometallic #uniBern
Reaction mechanism architect
Chemist, ICIQ Group Leader
Inorganic Chemistry @UniSaarland