
We are proud to collaborate with the Latin American Cooperative Oncology Group (LACOG) on innovative multicenter clinical studies like DORA and the IRONMAN Registry www.linkedin.com/embed/feed/u...
07.10.2025 16:52 — 👍 0 🔁 0 💬 0 📌 0@thepcctc.bsky.social
The Prostate Cancer Clinical Trials Consortium (PCCTC) translates scientific discoveries to improved standards of care. Visit pcctc.org to learn more.

We are proud to collaborate with the Latin American Cooperative Oncology Group (LACOG) on innovative multicenter clinical studies like DORA and the IRONMAN Registry www.linkedin.com/embed/feed/u...
07.10.2025 16:52 — 👍 0 🔁 0 💬 0 📌 0PCCTC investigators publish results from the phase 1b DeLLpro-300 study of tarlatamab in patients with neuroendocrine prostate cancer: aacrjournals.org/clincancerre...
01.10.2025 16:20 — 👍 0 🔁 0 💬 0 📌 0Prognostic Significance of PSA>0.2 After 6-12 Months Treatment for mHSPC Intensified by ARPI: A Multinational Real-World Analysis of the IRONMAN Registry. Story via UroToday: www.urotoday.com/conference-h...
24.09.2025 18:17 — 👍 0 🔁 0 💬 0 📌 0
PCCTC investigator Rana McKay, MD, outlines key findings from the phase 2 COMRADE trial via Urology Times
www.urologytimes.com/view/rana-mc...

Consensus Guidelines Needed to Further Optimize PARP Inhibition, Molecular Imaging, and ARPI Use in Prostate Cancer Management
@ucsdhealth.bsky.social #ProstateCancer #PCSM
www.onclive.com/view/consens...
Such an honor and so much fun to join the Cancer Clear and Simple @uwhealth.bsky.social Carbone Cancer Center Podcast to discuss liquid biopsies in cancer!
cancer.wisc.edu/community-ou...

PCCTC investigator Michael A. Carducci, MD discusses consensus recommendations for PARP inhibitor use in metastatic prostate cancer with Alan Bryce, MD via UroToday: www.urotoday.com/video-lectur...
22.07.2025 14:35 — 👍 1 🔁 0 💬 0 📌 0
Learn more about the PCCTC-managed PROMISE, a registry of prostate cancer patients participating in a research study to learn how genetic differences can affect patient outcomes: www.prostatecancerpromise.org
18.07.2025 16:30 — 👍 1 🔁 0 💬 0 📌 0
Esteemed PCCTC investigator Dr Rana McKay discusses the exciting developments in biomarker-driven therapy for metastatic castration-sensitive prostate cancer via @onclive.bsky.social: www.onclive.com/view/emergin...
10.07.2025 14:11 — 👍 0 🔁 0 💬 0 📌 0
Mark Your Calendar! 🙌🏾🙌🏽🙌🏿 We’re headed to Lagos for the IRONMAN Conference from September 2nd through the 5th. This isn’t just an event, it’s a major milestone in global health and cancer research you won’t want to miss!
Register now: surveys.mayoclinic.org/jfe/form/SV_...

PCCTC investigator Dr Rana McKay and colleagues discuss personalizing treatment intensification in metastatic castration-sensitive prostate cancer via @onclive.bsky.social: www.onclive.com/view/persona...
02.07.2025 15:03 — 👍 0 🔁 0 💬 0 📌 0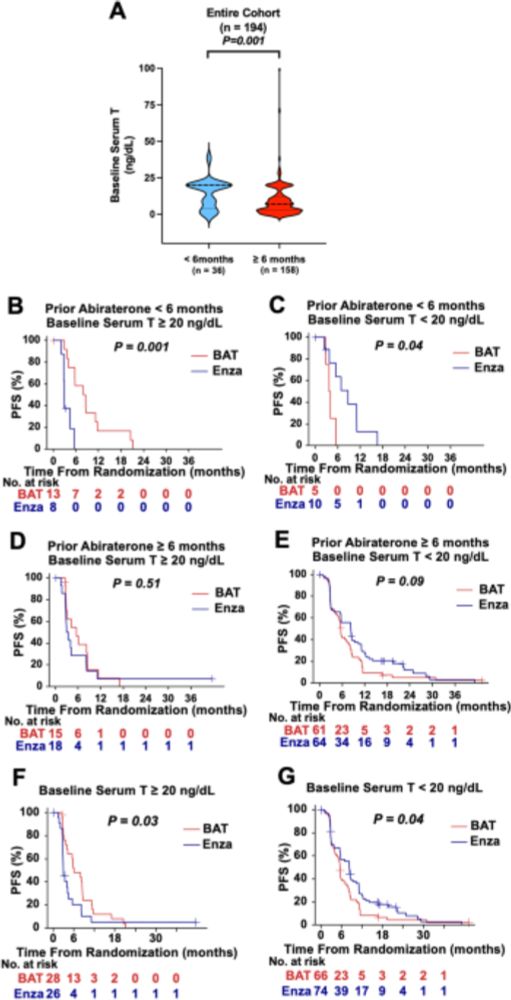
Just published: Analysis from the TRANSFORMER prostate cancer study reveals baseline testosterone could be considered in the treatment selection process when bipolar androgen therapy is an option www.nature.com/articles/s41...
02.07.2025 12:26 — 👍 0 🔁 0 💬 0 📌 0
From PCCTC investigator Dr Rana McKay and colleagues, "Radium-223 in Men with Metastatic Castration-resistant Prostate Cancer: A Systematic Literature Review of Real-world Outcomes in Observational Studies," published in Eur Urol Oncol www.sciencedirect.com/science/arti...
30.06.2025 17:58 — 👍 0 🔁 0 💬 0 📌 0Prognostic Significance of PSA>0.2 After 6-12 Months Treatment for Metastatic Hormone-Sensitive Prostate Cancer Intensified by Androgen-Receptor Pathway Inhibitors: A Multinational Real-World Analysis of the PCCTC-managed IRONMAN Registry www.urotoday.com/conference-h...
26.06.2025 13:04 — 👍 0 🔁 0 💬 0 📌 0Learn more about TALENT, the PCCTC-managed study of talazoparib with or without enzalutamide in people with #prostatecancer who have previously received abiraterone (NCT06844383): www.pcctc.org/talent
16.06.2025 23:38 — 👍 0 🔁 0 💬 0 📌 0#ASCO25: PCCTC investigator Dr Rana McKay discusses the COMRADE study, "The combination of olaparib with radium-223 demonstrated feasibility, safety, and antitumor activity in patients with metastatic castration-resistant #prostatecancer with bone metastases”
03.06.2025 21:20 — 👍 2 🔁 0 💬 0 📌 0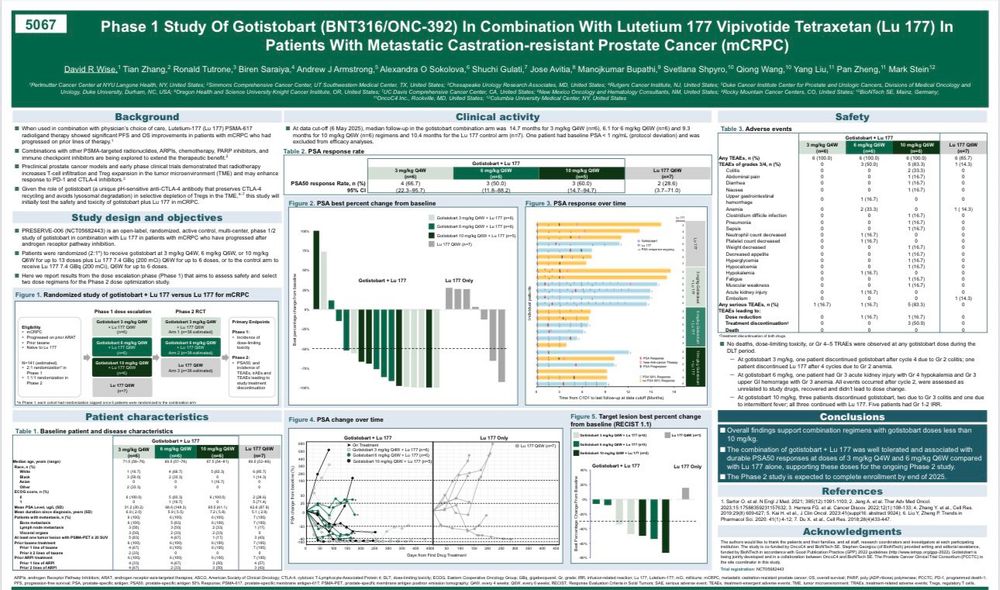
PCCTC study of a novel CTLA-engaging IO plus Lu177-PSA-617 therapy in patients with metastatic castration-resistant #prostatecancer (mCRPC) at #ASCO25 #MedIQASCO25 with @tiansterzhangmd.bsky.social. Clear activity and safe!
03.06.2025 13:30 — 👍 3 🔁 1 💬 0 📌 1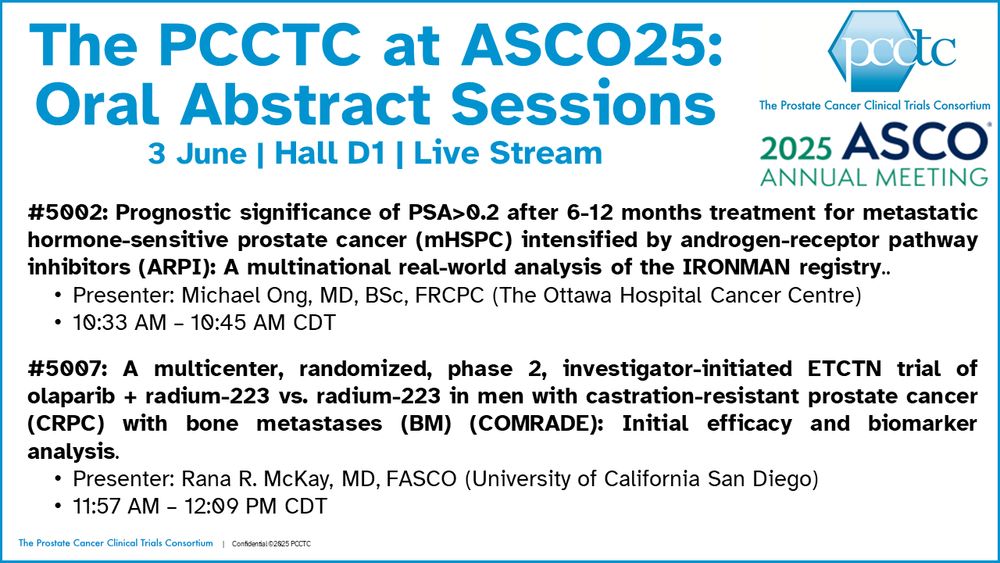

Join us tomorrow (Tuesday) at #ASCO25 as PCCTC investigators present at the prostate cancer oral abstract session (Hall D1 and Live Stream)!
02.06.2025 16:11 — 👍 0 🔁 0 💬 0 📌 0#ASCO25: PCCTC investigators Drs Karen Autio, Michael Schweizer, and Mark Stein contribute to first-in-human results for novel T-cell engager pasritamig (JNJ-78278343), showing early anti-tumor activity in #prostatecancer www.jnj.com/media-center...
01.06.2025 14:19 — 👍 2 🔁 0 💬 0 📌 0The PCCTC at #ASCO25 via UroToday: A Randomized, Open-Label, Phase 2b Study of the Bromodomain Inhibitor ZEN-3694 + Enzalutamide vs Enzalutamide in Patients with mCRPC www.urotoday.com/conference-h...
01.06.2025 14:04 — 👍 1 🔁 0 💬 0 📌 0
Today at #ASCO25, Rapid Oral Sessions featuring PCCTC #prostatecancer science: the phase 2 Metacure trial, the novel B7H3 ADC DB‑1311/BNT324, and the phase 1 study results of JNJ-78278343
Today at #ASCO25, Rapid Oral Sessions featuring PCCTC #prostatecancer science: the phase 2 Metacure trial, the novel B7H3 ADC DB‑1311/BNT324, and the phase 1 study results of JNJ-78278343
01.06.2025 13:04 — 👍 2 🔁 0 💬 0 📌 0
Mark Your Calendar! 📆 We’re headed to Lagos for the IRONMAN Conference from September 2nd through the 5th. This isn’t just an event, it’s a major milestone in global health and cancer research you won’t want to miss!
Register now: surveys.mayoclinic.org/jfe/form/SV_...

DOD prostate cancer research program FY25 funding opportunities
https://cancerletter.com/funding-opportunities/20250523_10a/

The Prostate Cancer Clinical Trials Consortium at ASCO 2025: Abstracts Listing
#ASCO25 is just days away. Be sure to check out the innovative PCCTC prostate cancer clinical research presented at this year's annual meeting in Chicago!
28.05.2025 18:38 — 👍 2 🔁 0 💬 0 📌 0
The PCCTC at ASCO25: Oral Abstract Sessions 3 June | Hall D1 | Live Stream #5002: Prognostic significance of PSA>0.2 after 6-12 months treatment for metastatic hormone-sensitive prostate cancer (mHSPC) intensified by androgen-receptor pathway inhibitors (ARPI): A multinational real-world analysis of the IRONMAN registry.. Presenter: Michael Ong, MD, BSc, FRCPC (The Ottawa Hospital Cancer Centre) 10:33 AM – 10:45 AM CDT #5007: A multicenter, randomized, phase 2, investigator-initiated ETCTN trial of olaparib + radium-223 vs. radium-223 in men with castration-resistant prostate cancer (CRPC) with bone metastases (BM) (COMRADE): Initial efficacy and biomarker analysis. Presenter: Rana R. McKay, MD, FASCO (University of California San Diego) 11:57 AM – 12:09 PM CDT

The PCCTC at ASCO25: Rapid Oral Abstract Sessions 1 June | Hall D2 | Live Stream #5014: Intensified hormonal blockade with SBRT in PSMA-PET detected oligometastatic prostate adenocarcinoma: Results from the phase II Metacure trial cohorts B2 and the B2 expansion. Presenter: Eric Huttenlocher Bent, MD, PhD (MSK) 4:42 PM – 4:48 PM CDT #5015: DB‑1311/BNT324 (a novel B7H3 ADC) in patients with heavily pretreated castrate-resistant prostate cancer (CRPC). Presenter: Andrew Ohyama Parsonson, FRACP, MBBS (Macquarie University) 5:00 PM – 5:06 PM CDT #5017: Phase 1 study results of JNJ-78278343 (pasritamig) in metastatic castration-resistant prostate cancer (mCRPC). Presenter: Capucine Baldini, MD, PhD (Institut Gustave Roussy) 5:12 PM – 5:18 PM CDT

The PCCTC at ASCO25: Poster Sessions Gupta S, et al. Does cytoplasmic AR-V7 circulating tumor cell (CTC) detection add utility in predicting AR pathway inhibitor benefit in men with mCRPC? A retrospective analysis of the PROPHECY study. Abstract # 5043, Poster Bd # 242. Von Amsberg G, et al. Phase 1b/2 KEYNOTE-365 cohort I: Pembrolizumab (pembro) plus carboplatin and etoposide chemotherapy (chemo) or chemo alone for metastatic neuroendocrine prostate cancer (NEPC). Abstract # 5059, Poster Bd #258. Marulanda Corzo V, et al. PSMA-targeted actinium-225 therapy in metastatic castration-resistant prostate cancer (mCRPC): Baseline and follow-up PSMA PET parameters associated with outcomes. Abstract # 5070, Poster Bd # 269. Bryce A, et al. Preliminary phase 2 results of PT-112 monotherapy in late-line metastatic castration-resistant prostate cancer (mCRPC). Abstract # 5071, Poster Bd # 270. Attwell M, et al. A randomized, open-label, phase 2b study of the BET bromodomain inhibitor (BETi) ZEN-3694 plus enzalutamide vs. enzalutamide in patients with metastatic castration resistant prostate cancer (mCRPC). Abstract # TPS5123, Poster Bd # 315b. Wise D, et al. Phase 1 study of gotistobart (BNT316/ONC-392) in combination with lutetium Lu 177 vipivotide tetraxetan (Lu 177) in patients with metastatic castration-resistant prostate cancer (mCRPC). Abstract # 5067, Poster Bd #266. Johnson G, et al. SECuRE: A dose escalation/expansion study to assess the anti-tumor efficacy of 67Cu-SAR-bisPSMA in patients with metastatic castrate resistant prostate cancer. Abstract # TPS5125, Poster Bd # 316b.

The PCCTC at ASCO25: Publication Only Abstracts Sokolova A, et al. Barriers to germline genetic testing in advanced prostate cancer: Survey results from the Germline Genetics Working Group of the Prostate Cancer Clinical Trials Consortium (PCCTC). Abstract # e17073. George D, et al. Predictive associations between serum dehydroepiandrosterone sulfate (DHEAS) and race among patients (pts) treated with apalutamide (apa), abiraterone acetate (AA) plus prednisone (P) in the PANTHER study. Abstract # e17076. Schweizer M, et al. Modulation of enhancer of zeste homolog 2 (EZH2) pharmacodynamic (PD) markers and tumor gene expression by mevrometostat in combination with enzalutamide in patients with castration-resistant prostate cancer (CRPC). Abstract # e17032. Alonso Gordoa T, et al. Mevrometostat, an enhancer of zeste homolog 2 (EZH2) inhibitor, in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer (mCRPC): A randomized dose-expansion study. Abstract # e17029.
We are excited to announce science from innovative PCCTC multi-center prostate cancer studies will be represented by 16 abstracts at #ASCO25!
22.05.2025 21:05 — 👍 1 🔁 0 💬 0 📌 0
At MSK, there are exciting new therapies that are proving effective at treating metastatic prostate cancer.
In this 📹, Dr. Michael Morris, a genitourinary medical oncologist and the Prostate Cancer Section Head at MSK, speaks about one in particular.
Plus, read more here: bit.ly/4mfOKhg
The PCCTC's Dr. Michael J. Morris and other experts weigh in on President Biden's prostate cancer diagnosis and the debate about prostate screenings for older men: www.washingtonpost.com/health/2025/...
19.05.2025 21:27 — 👍 2 🔁 0 💬 0 📌 0
We’re headed to Lagos for the IRONMAN Conference from September 4–6, with a special pre-conference session on September 3. This isn’t just an event—it’s a major milestone in global health and cancer research you won’t want to miss!
Register now: surveys.mayoclinic.org/jfe/form/SV_...
Cover page of Olaparib and Durvalumab in Patients with DNA Damage Repair Alterations and Biochemically Recurrent Prostate Cancer
Just published: PCCTC study of Olaparib and Durvalumab in Patients with DNA Damage Repair Alterations and Biochemically Recurrent Prostate Cancer by Dr Karen A Autio and colleagues: pmc.ncbi.nlm.nih.gov/articles/PMC...
02.05.2025 18:05 — 👍 0 🔁 0 💬 0 📌 0
Recommended Clinical Context and Patient Context Data Elements for Liquid Biopsy Data Submitted to Data Repositories and Data Commons: In 2020, BLOODPAC recommended 11 pre-analytical minimal technical data elements for collection and submission of liquid biopsy data to public databases. This article expands on that work by recommending 22 clinical context and 10 patient context data elements. These elements, essential for liquid biopsy data submitted to repositories like the BLOODPAC Data Commons, cover tumor characteristics, disease progression, and patient demographics, supporting biomarker validation, research, and clinical trials.
Just Published: PCCTC leadership and colleagues release recommended clinical context and patient context data elements for liquid biopsy data submitted to data repositories and data commons (Clin Transl Sci): pmc.ncbi.nlm.nih.gov/articles/PMC...
01.05.2025 13:02 — 👍 0 🔁 0 💬 0 📌 0