
Excellent safety letter in #OrgLettAsap today from the Garg Lab (UCLA) and #MerckChemistry on the hazards of IBX - please read
And thank you to Org Lett for publishing this informative article
#ChemSky
@agomezsuarez.bsky.social
Chemist. PI of the Gómez-Suárez lab (radical.uni-wuppertal.de) and scientific staff of the Kirsch group (oc-sfk.uni-wuppertal.de) at the Bergische Universität Wuppertal.

Excellent safety letter in #OrgLettAsap today from the Garg Lab (UCLA) and #MerckChemistry on the hazards of IBX - please read
And thank you to Org Lett for publishing this informative article
#ChemSky

Stirring: friend or foe?
Huang et al.: 329 reactions → stirring made little difference.
Cherepanova et al.: stirrers can cause irreproducibility by vial position.
Time to rethink the humble stir bar.
doi.org/10.1021/jacs... #ChemSky

I’m proud to share that my group has received a Starting Grant from @erc.europa.eu, which will enable us to explore new organophotomediated reactions, and deeply grateful to my group and the many colleagues who helped with the application.
04.09.2025 13:51 — 👍 20 🔁 1 💬 2 📌 0
Congrats to @fruepp.bsky.social, Vasily Grebennikov, Mykola Avramenko, Marc-Olivier Ebert "Kinetic, Spectroscopic, and Computational Investigation of Oxidative Aminative Alkene Cleavage Reveals an N-Iodonium-Iminoiodinane Pathway" now @chemrxiv.org - chemrxiv.org/engage/chemr...
02.09.2025 19:18 — 👍 14 🔁 6 💬 1 📌 0
Congrats to Ann-Sophie Paschke, @ybraegger.bsky.social, Bence Botlik, Erich Staudinger, @origreen.bsky.social! "Carbon-to-nitrogen atom swap enables direct access to benzimidazoles from drug-like indoles". www.nature.com/articles/s41.... @natchem.nature.com.
02.09.2025 18:49 — 👍 22 🔁 3 💬 0 📌 1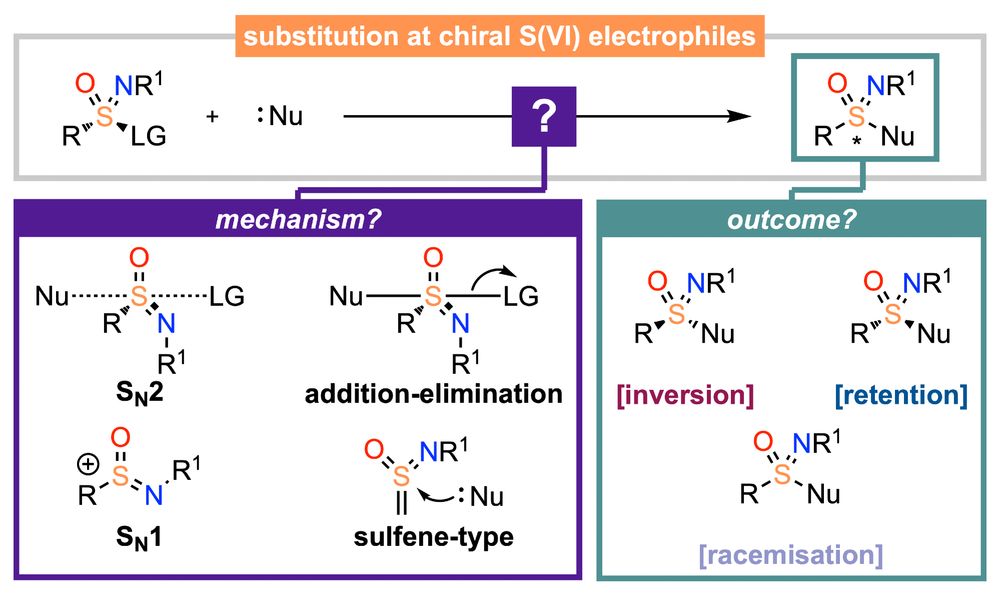
As asymmetric sulfur(VI) derivatives become more prevalent in the chemical sciences, especially in aza-derivatives, sulfoximines, sulfonimidamides, we have reviewed "The stereochemistry of substitution at S(VI)"
doi.org/10.1039/D5QO...
with Ollie Symes, @orgchemfront.rsc.org

Do Amino-Oxetanes Resemble Amides?
Check out our Matched Molecular Pairs study in
@chemrxiv.org comparing properties and structure.
Congrats @hikaruishikura.bsky.social and coworkers, in collaboration with Pfizer.
doi.org/10.26434/che...
Many Thanks for the wonderful highlight!!
20.08.2025 06:31 — 👍 6 🔁 1 💬 0 📌 0
Today after years of work and a tight collaboration between >40 chemists in academia and industry, we are thrilled to share the story of how our Advanced Organic Chemistry course and other courses were created in the Journal of Chemical Education!
Link: doi.org/10.1021/acs....

📢pls share
We are hiring! New opening for a W2 Professor in "experimental inorganic chemistry" @unibonn.bsky.social
Deadline Oct. 10 t.co/EqMLA0fCuC

Liu & Cole use neural networks on 50k+ ¹³C/¹H NMR spectra with metadata to predict molecular substructures. Their MLP+LSTM model hit 88% accuracy; CNNs reached 86% in one-third the time, enabling fast, automated NMR analysis for chemistry and drug discovery. pubs.acs.org/doi/full/10....
16.08.2025 14:19 — 👍 8 🔁 4 💬 0 📌 0
SuFEx welcomes new coupling partners - carbon pronucleophiles now in @chemicalscience.rsc.org . Congrats to Ball Lab students Joe Novicku and Matt Teeter and Pfizer colleagues Alistair, Tom, Neil, ans @chrisamende.bsky.social on the great work! #MyFirstChemSci
pubs.rsc.org/en/content/a...

No matter how hard you try, you simply cannot guess the journal withe the highest “impact factor” in organic chemistry last year. Really. Nor will you guess many of the other “winners”:
14.08.2025 18:32 — 👍 77 🔁 26 💬 14 📌 12
🔓Check out this #OpenAccess review by Yasushi Nishihara & co. from Okayama University in our latest issue looking at modern decarboxylative approaches for sustainable C–N and C–O bond formation #organicchemistry
Read the full article on our website🔽
pubs.rsc.org/en/content/a...

Now published in ChemistryEurope: doi.org/10.1002/ceur...
13.08.2025 10:26 — 👍 3 🔁 3 💬 0 📌 0Excited that our group was awarded an NWO XS grant to support our research into new #covalent #antibiotics for Gram-negative bacteria!
Thank you to Storm van der Voort for help with the proposal.
Congratulations also to all other recipients of the grant!
www.nwo.nl/en/news/48-g...
#ChemSky #ChemBio
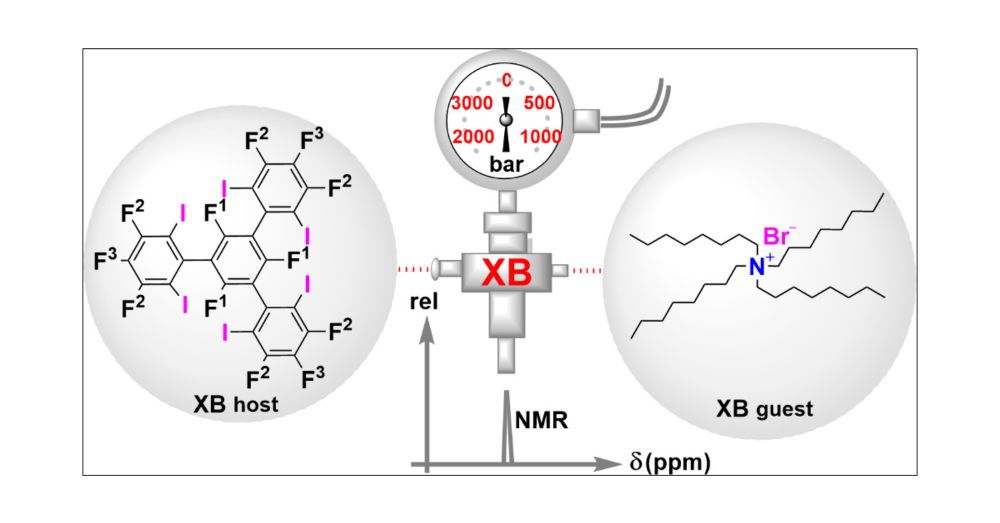
A paper with a Queen song title? Check! (Even though that was an easy one).
We've investigated the high pressure behavior of halogen bonding and found some unexpected solvent @solvationsci.bsky.social dependence:
pubs.acs.org/doi/10.1021/...

Author profile in Helvetica
Today is the 1st of August, National day of Switzerland and I am happy to share my profile author in @helvchimacta.bsky.social , as a part of their Early Career Board. Have a read if you want to know a bit more about me 😊 doi.org/10.1002/hlca... and happy 1st of August 🇨🇭
01.08.2025 08:43 — 👍 18 🔁 4 💬 1 📌 0
#PhDposition #OrganicChemistry #ChemBio - deadline 03.08 - please RT ❤️ Application online stellenangebote.uni-marburg.de/jobposting/d...
29.07.2025 12:29 — 👍 9 🔁 10 💬 0 📌 0A postdoctoral position, funded by @leverhulme.ac.uk for up to 36 months, is available to join my group @edinburghchem.bsky.social. The project focuses on biomimetic approaches to natural product synthesis.
Deadline: 28th August 2025.
elxw.fa.em3.oraclecloud.com/hcmUI/Candid...

JOB ALERT! 🚨 Are you interested in organic synthesis and catalysis? We have an open PhD position in our group at Philipps-Universität Marburg in Germany. 👩🔬👨🔬 Deadline: 10th August 2025. For more details and to submit an application, please refer to the official posting here: lnkd.in/e_f3Xerq
#chemsky
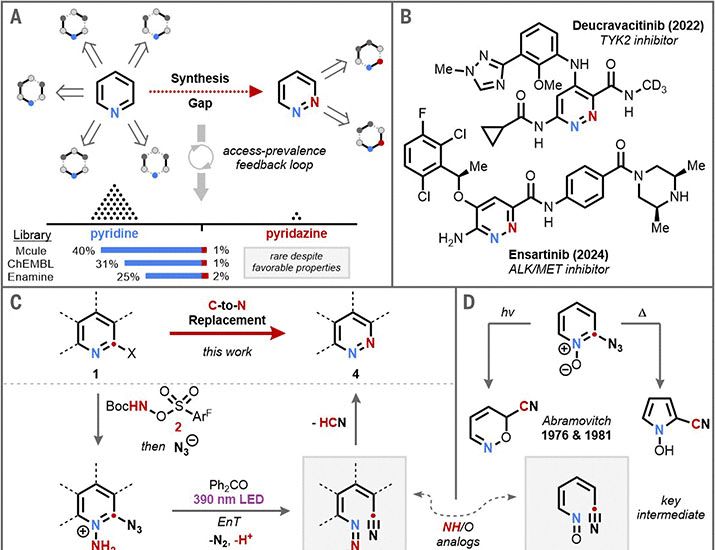
It’s pyridazine day at @science.org as two groups make double-N hexagonal rings! @levinchem.bsky.social and @mikusp.bsky.social do it by orchestrating an N-for-C swap in pyridines
chemsky 🧪
www.science.org/doi/10.1126/...
Meanwhile Hongjian Lu’s group insert nitrogen into 5-membered pyrrolidines
chemsky 🧪
www.science.org/doi/10.1126/...
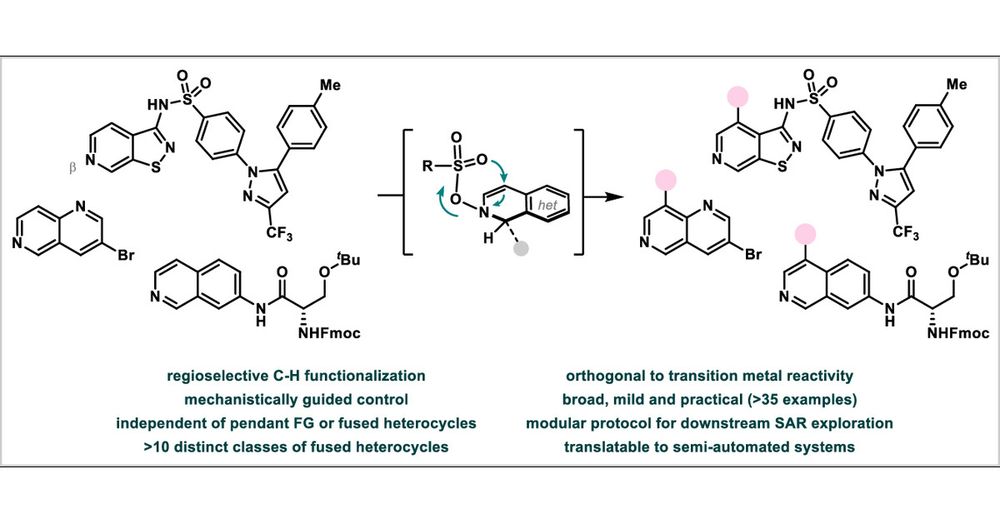
Check out this new direct #CHfunctionalization method for fused azines at #ACScentralscience : broad, metal-free and proceeding with exclusive and predictable #regiocontrol! Executed in #parallelmode for its immediate application to #drugdiscovery! 💊
pubs.acs.org/doi/10.1021/...
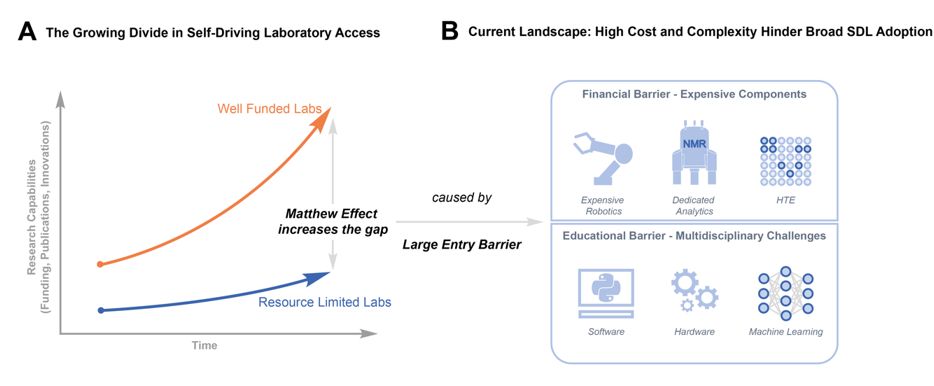
🚫 Current self-driving labs (SDLs) cost >$100K, blocking access for most researchers.
💡 Enter RoboChem-Flex: A fully modular, AI-driven lab for under €5K.
Built to democratize automation in chemistry. Open, affordable, and powerful.
🔗 chemrxiv.org/engage/chemr...
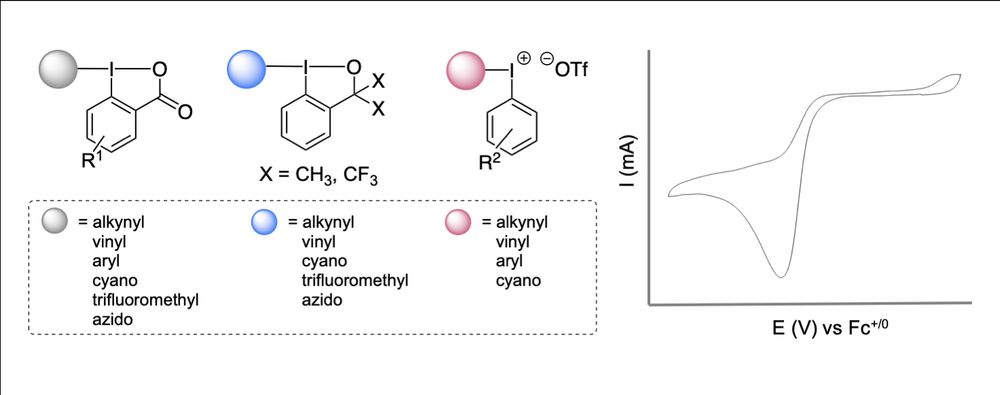
Our paper on the synthesis of >50 iodine reagents, and the systematic analysis of their electrochemical properties, is just out in Synthesis @synthesis1969.bsky.social 🤩
⚠️Full disclosure: this is my very first paper without transition metals! 😜 read it here: www.thieme-connect.com/products/ejo...

New @chemrxiv.bsky.social preprint!
RoboChem-Flex is a powerful, low-cost (<5k EUR), modular self-driving lab for chemical synthesis
We showcase 6 studies (photochemistry, biocatalysis, cross coupling, ee ...), all optimized with different configurations & ML
🔗 chemrxiv.org/engage/chemr...
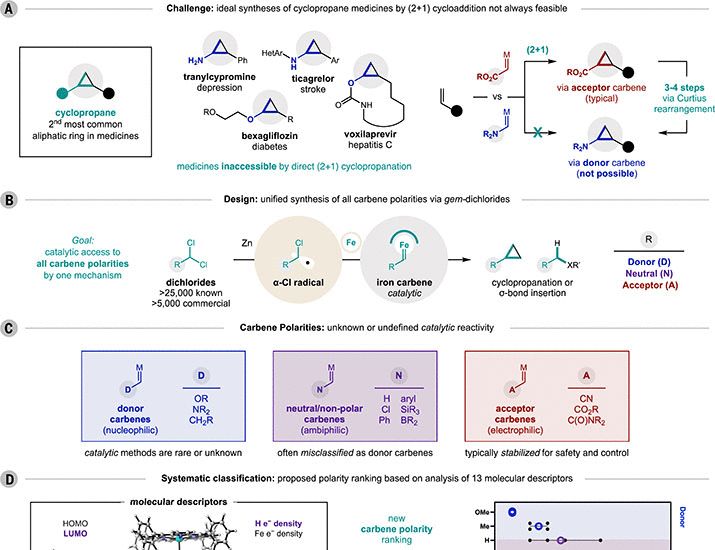
In @science.org this week, THE @nagiblab.bsky.social makes all the carbenes (no, seriously!) and classifies their electronic properties on a common basis
chemsky 🧪
www.science.org/doi/10.1126/...