
Another contribution to photochemical deracemization! Check out Max' outstanding work on chiral phosphoric acids, out now in @chemicalscience.rsc.org! 💡🧑🔬🧪
#photochemistry #CPA #deracemization
doi.org/10.1039/D5SC...
@bach-lab.bsky.social
Photocatalysis, Supramolecular Catalysis, Total Synthesis, and C-H Activation (student run account)

Another contribution to photochemical deracemization! Check out Max' outstanding work on chiral phosphoric acids, out now in @chemicalscience.rsc.org! 💡🧑🔬🧪
#photochemistry #CPA #deracemization
doi.org/10.1039/D5SC...
Another recent most read article, from @bach-lab.bsky.social
'Photocatalytic reductive incorporation of carbon dioxide into double bonds' 🔓


Congratulations to our amazing soccer team for winning the championship of the @tum.de NAT tournament! Despite heavy rain in the end, you kept fighting and brought the trophy back home. 🏆🥇⚽ #Soccer #TUM #winner
17.07.2025 14:07 — 👍 2 🔁 0 💬 0 📌 0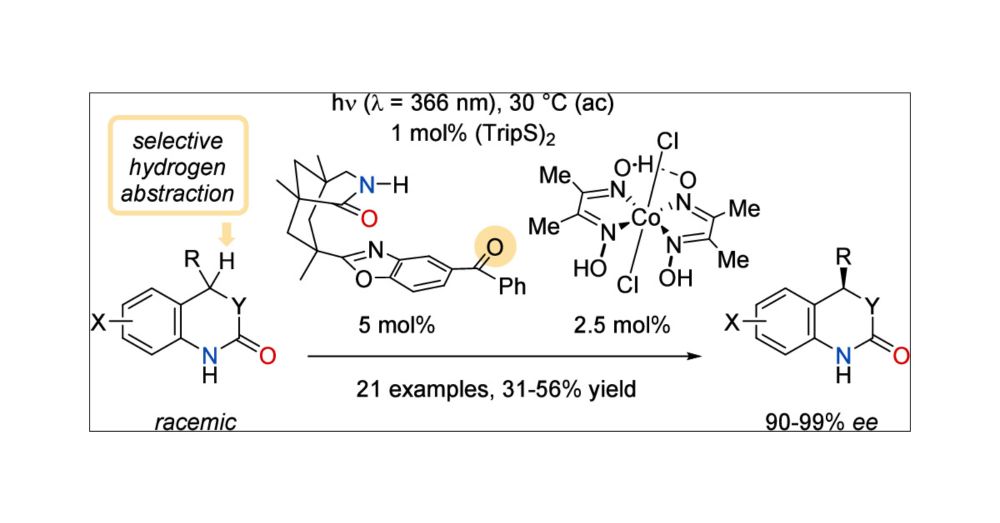
Great news! Check out our latest paper on the co-catalytic photochemical kinetic resolution by dehydrogenation. Amazing work, Chao! 💡🙌🧪 #photochemistry #dehydrogenation #kineticresolution
doi.org/10.1021/jacs.5c07524


What an amazing day at the Campus Run 2025! 🏃♀️🏃♂️
So proud of everyone who took part! Huge participation and great energy all around.
Special shoutout to Simone for finishing 3rd fastest woman over 11 km! 🔥
Big thanks to our awesome cheer squad 🫶 You really made a difference! 💙💪
#CampusRun #Race #TUM
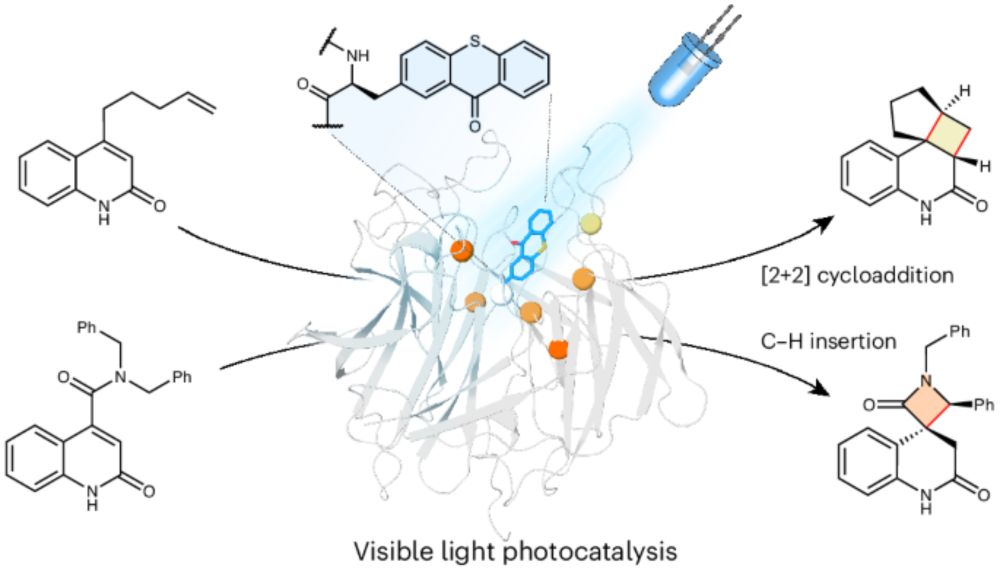
Amazing work, Johannes! Check out his contribution to the work of the Green Group from @uommib.bsky.social on ennatioselective photoenzymatic C-H insertion of quinolones, out now in @natchem.nature.com! #photochemistry #photoenzyme #enantioselective💡🧬🥳
www.nature.com/articles/s41...

First defense of the year: Congratulations to freshly minted Dr. Niklas Pflaum! Excited to have you for a few more months with us #PhD #graduation 🎓👏💡
28.04.2025 10:17 — 👍 3 🔁 0 💬 0 📌 0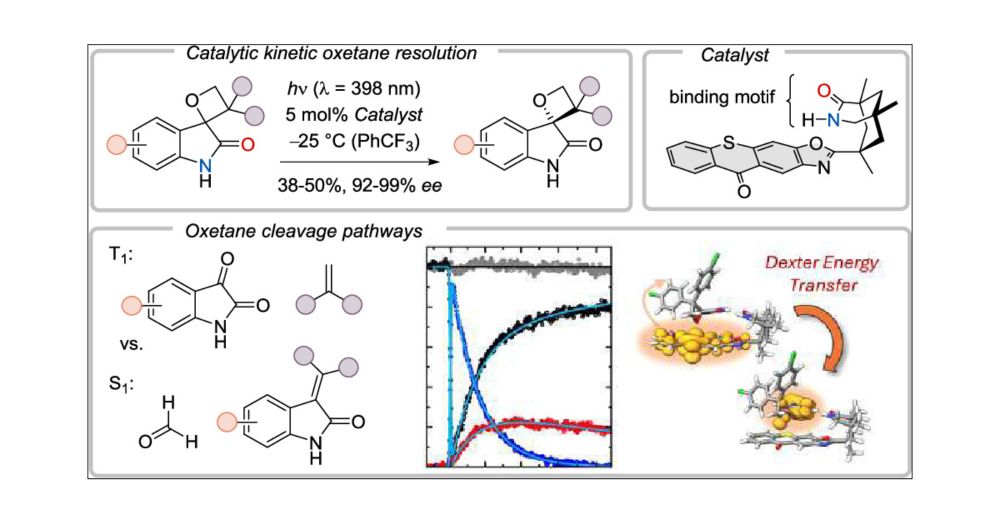
Excited to share the news: Niklas' paper on enantiopure oxetanes is out now in @jacs.acspublications.org. Great work by all and big thanks to all collaboration partners! 💡👏 #photochemistry #enantioselectivity
doi.org/10.1021/jacs...



Happy 60th birthday, Thorsten! 🎂🍾🎈 Thank you for being an amazing PI, for all your support and motivation. We hope there will be many more happy occasions to celebrate with you in the future. We wish you all the best for your next 60 years 😇😁 #birthday #celebration #60years
15.04.2025 10:57 — 👍 4 🔁 0 💬 0 📌 0
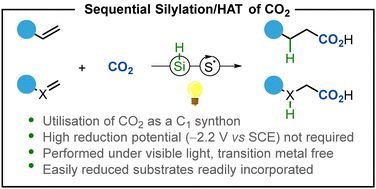
Interested in photochemical activation of carbon dioxide? Check out Mark's work by using sequential silylation/HAT of CO2! Out now in Chem. Commun. by @rsc.org. #photochemistry #carbondioxide 💡🫧
pubs.rsc.org/en/content/a...

New paper out now in @angewandtechemie.bsky.social! Congratulations Max on your successful project on photochemically induced Diels-Alder reactions of twisted cycloheptenones. Big thanks also to all collaboration partners! #photochemistry #enantioselective 💡👏
onlinelibrary.wiley.com/doi/10.1002/...

Welcome Veronika to our group! 🙌 Good luck for your Master's thesis on borylations. 👩🔬💫 #WomeninSTEM #catalysis
25.03.2025 09:43 — 👍 4 🔁 0 💬 0 📌 0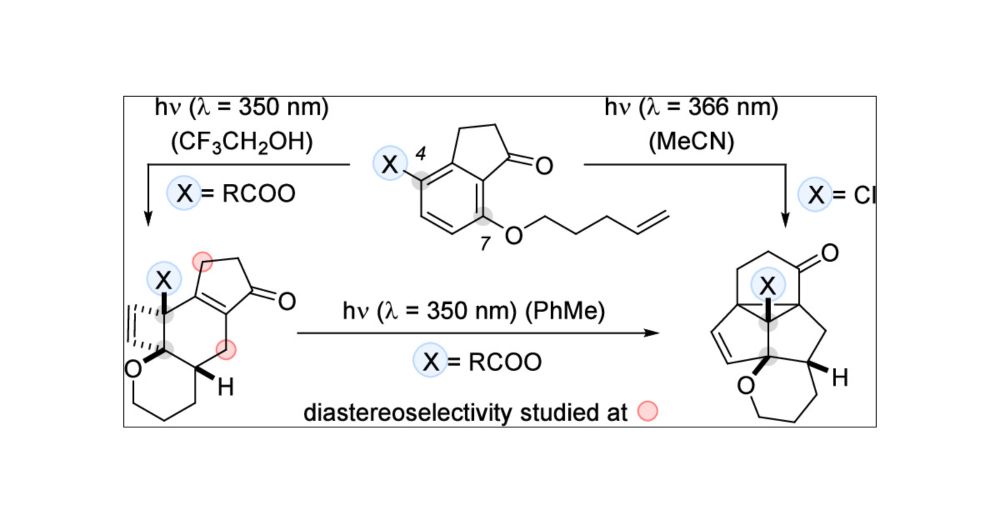
Check out Audrey's work on ortho photocycloaddition reaction cascades published in JOC! #Photochemistry #WomeninSTEM 💡👩🔬
pubs.acs.org/doi/full/10....

We're very excited to have Dr. Charlotte Teschers as a Postdoc with a return grant in our lab. 🌟 We hope you enjoy your time with us and good luck for your future career as an independent researcher! 👩🔬🙌 #WomeninSTEM #chemistry
27.02.2025 15:46 — 👍 3 🔁 0 💬 0 📌 0
Another new member to the group: Welcome Nina! We wish you all the best for your PhD. #womeninstem👩🎓🔬
26.02.2025 15:20 — 👍 2 🔁 1 💬 0 📌 0
A big welcome to Florian who joined us for his PhD in February. Good luck for your project on photochemistry! 🧑🔬🎓
26.02.2025 08:01 — 👍 1 🔁 0 💬 0 📌 0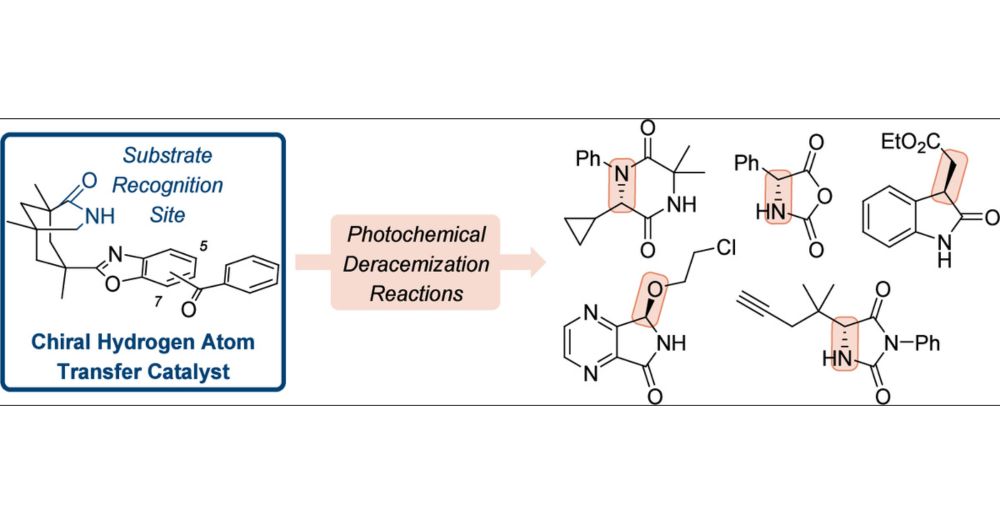
Interested in stereochemical editing by photochemically triggered hydrogen atom transfer? Check out our latest review by Maxi 💡🧪
pubs.acs.org/doi/10.1021/...
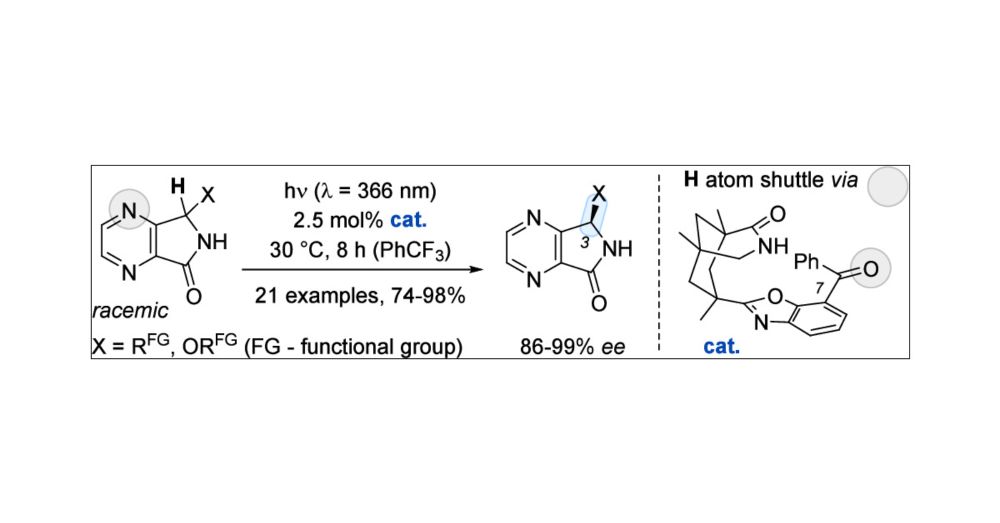
Let's start our year with a new publication in #JACS: Check out Philip's work on photochemical deracemization of 4,7-diaza-1-isoindolinones. #Photochemistry #Deracemization💡
pubs.acs.org/doi/10.1021/jacs.4c16053

AK Bach goes Bluesky! We are excited to share our journey on photochemistry here. Follow us to stay updated!💡🥼 #Photochemistry #Organicchemistry #Science #Chemsky
ch.nat.tum.de/oc1