N‐methylbicyclo[2.2.1]hept‐2‐ene‐2‐carboxamide
[2394994-09-1] C9H13NO (MW 151.21) InChI = 1S/C9H13NO/c1-10-9(11)8-5-6-2-3-7(8)4-6/h5-7H,2-4H2,1H3,(H,10,11) InChIKey = BYXAMVZFVXOYIF-UHFFFAOYSA-N (reagent used as a co-catalyst...
Proud to add one more reagent to EROS!
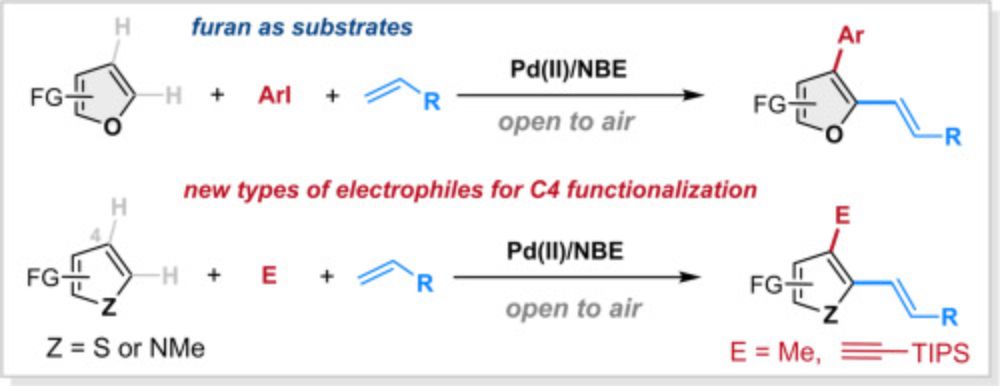
In this article, we summarized the synthesis and application of the N-methylbicyclo[2.2.1]hept-2-ene-2-carboxamide, a co-catalyst in the palladium/norbornene cooperative catalysis.
Congrats to Dr. Shinyoung Choi!
onlinelibrary.wiley.com/doi/10.1002/...
03.07.2025 19:13 — 👍 2 🔁 1 💬 0 📌 0
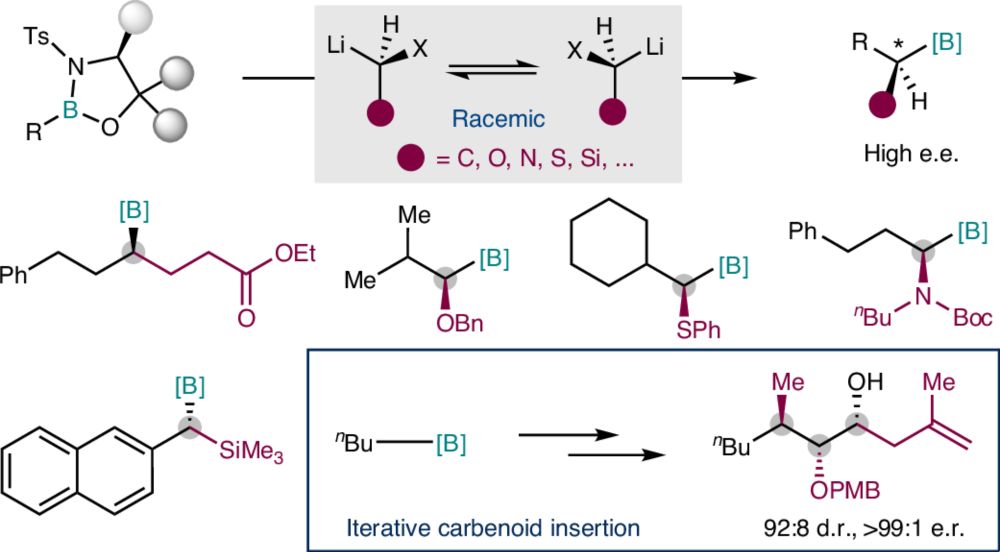
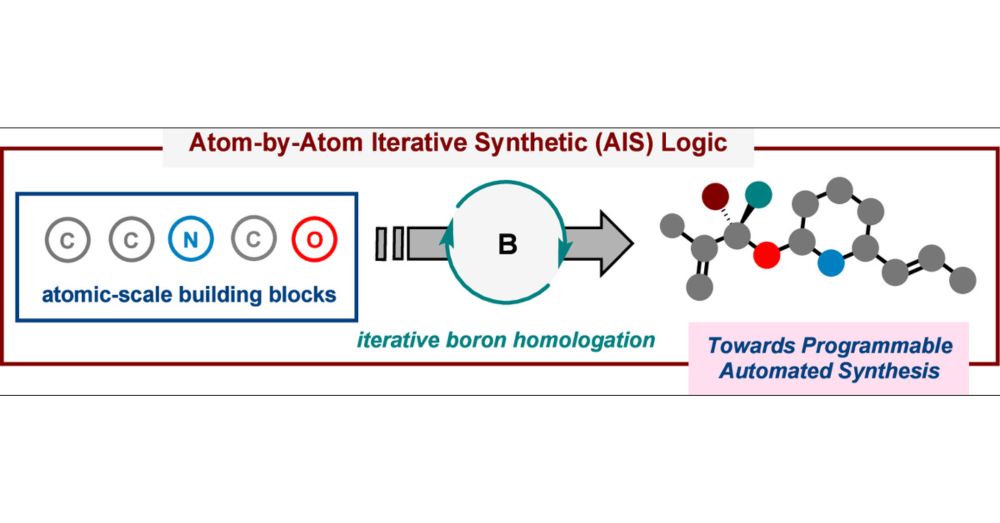
Enantioconvergent carbenoid insertion into carbon–boron bonds
Nature Synthesis - An enantioconvergent approach for direct asymmetric insertion of racemic carbon-, oxygen-, nitrogen-, sulfur- and silicon-substituted carbenoids into carbon–boron bonds is...
🔥 Scalable achievement in enantioselective homologation.
🔥 Our work on "Enantioconvergent carbenoid insertion into carbon−boron bonds" is now online on Nature Synthesis rdcu.be/etopg
🎉Congratulations to Qiqiang!
🙏Thanks to our collaborator Liu group
25.06.2025 18:50 — 👍 4 🔁 2 💬 0 📌 0
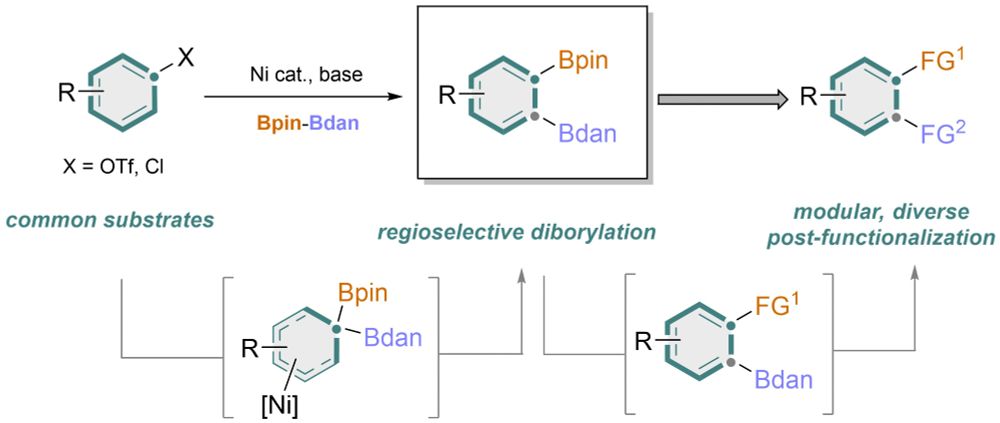
🚨Just out in Nature!
We have offered a general method for 1,2-difunctionlization of arenes via a differential 1,2-diborylation!
rdcu.be/esXq8
www.nature.com/articles/s41...
Congratulations to Jingfeng!
23.06.2025 21:59 — 👍 22 🔁 5 💬 0 📌 0
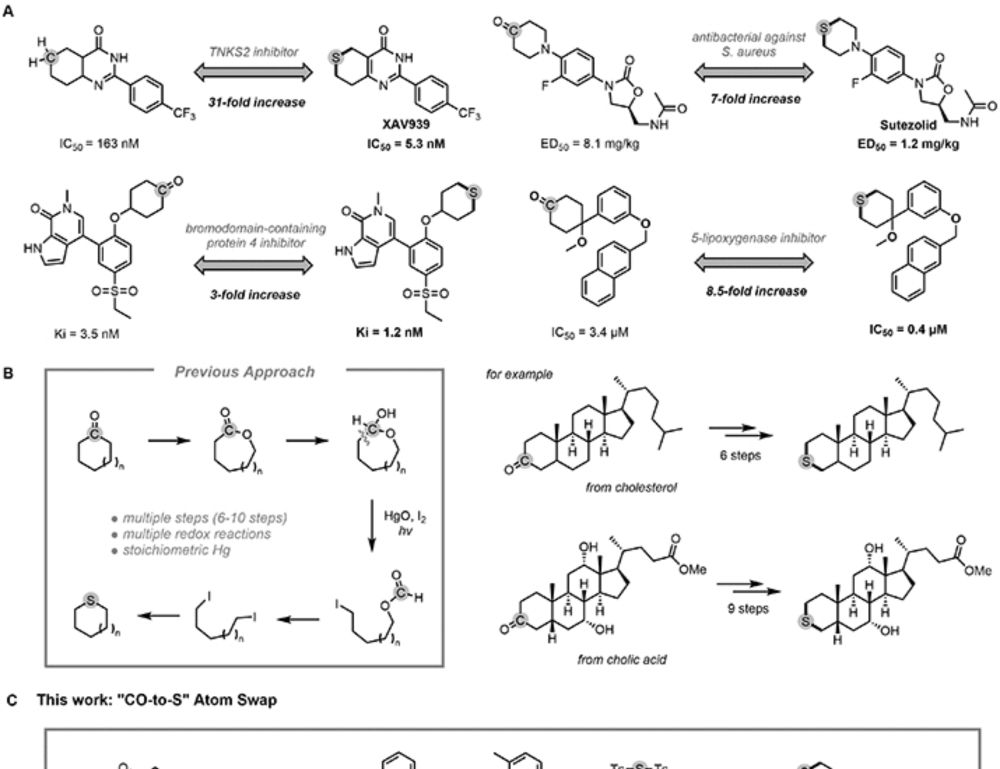
How do you turn a carbonyl into sulfur?
In this work, Zining from our lab developed a carbonyl-to-sulfur swap enabled by a rationally designed N′-alkyl-hydrazonamide (NAHA) reagent that promotes double C-C bond activation.
www.science.org/doi/10.1126/...
12.06.2025 21:13 — 👍 30 🔁 12 💬 0 📌 0
A big thanks to all the authors and the incredible Pd/NBE community for pushing the boundaries of this powerful platform. Looking forward to many more innovative and impactful transformations in the future!
19.04.2025 01:22 — 👍 2 🔁 0 💬 0 📌 0

Excited to share that “Palladium and Norbornene Cooperative Catalysis: Fundamentals and Applications”—edited by Guangbin—is now available via Wiley!
www.wiley.com/en-sg/Pallad...
19.04.2025 01:21 — 👍 3 🔁 1 💬 1 📌 0