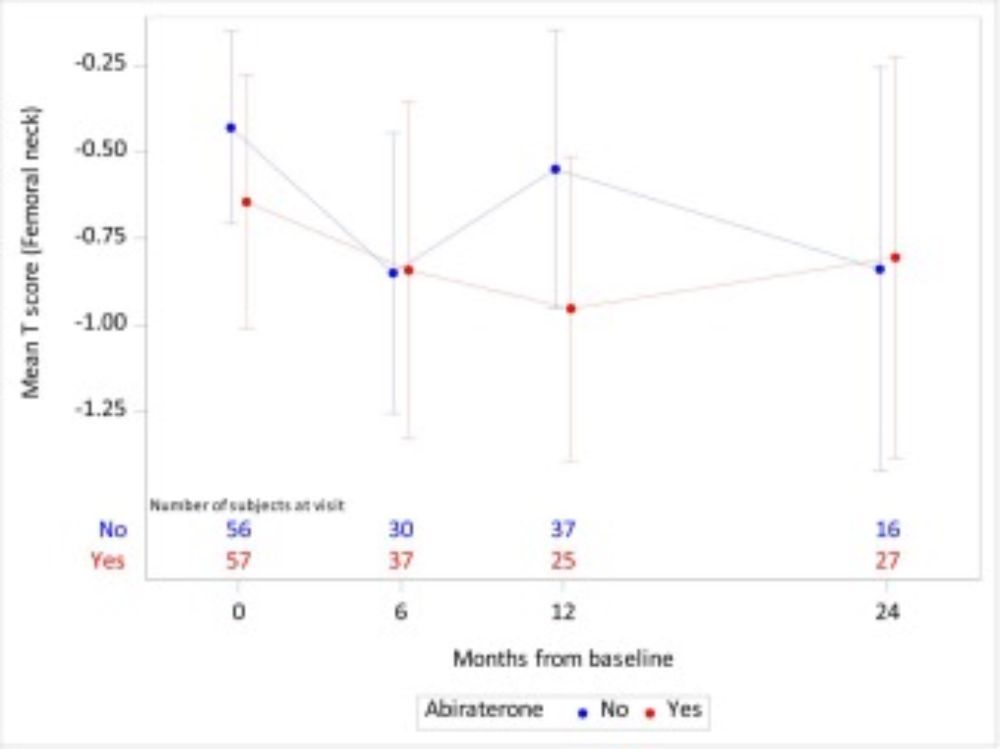
Decrease in Fracture Rate with Mandatory Bone-protecting Agents in the EORTC 1333/PEACE-3 Trial Comparing Radium-223 Combined with Enzalutamide Versus Enzalutamide Alone: A Safety Analysis - European Urology www.europeanurology.com/article/S030...
06.03.2025 18:01 — 👍 0 🔁 0 💬 0 📌 0
ASCO Publications
Accelerating the Future of Oncology Drug Development: The Role of Consortia in the Delivery of Precision Oncology Early Phase Clinical Trials. ascopubs.org/doi/10.1200/...
25.02.2025 07:57 — 👍 0 🔁 0 💬 0 📌 0
And that’s only in the case you’re “lucky enough” to get published!
28.12.2024 11:27 — 👍 1 🔁 0 💬 0 📌 0
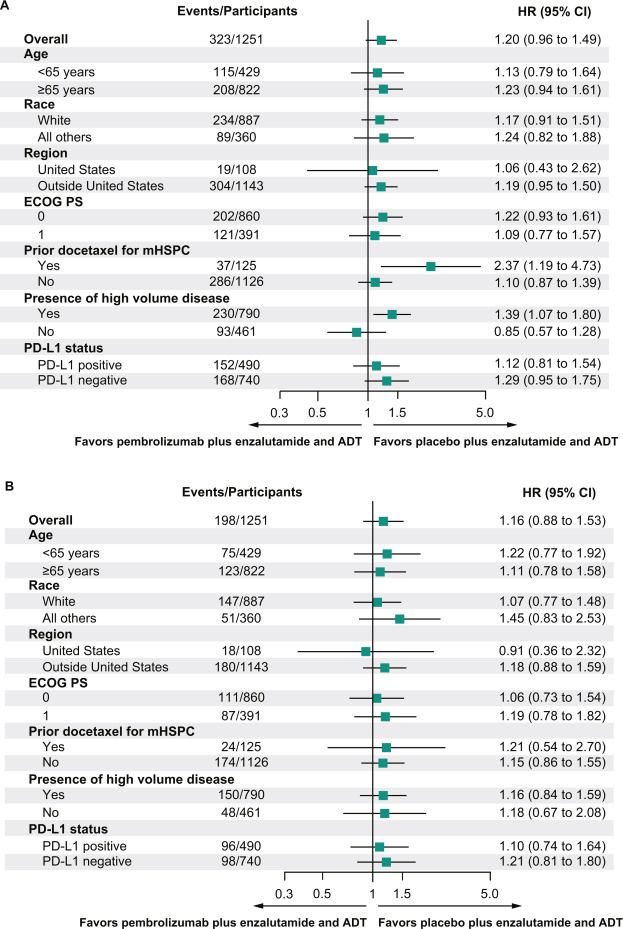
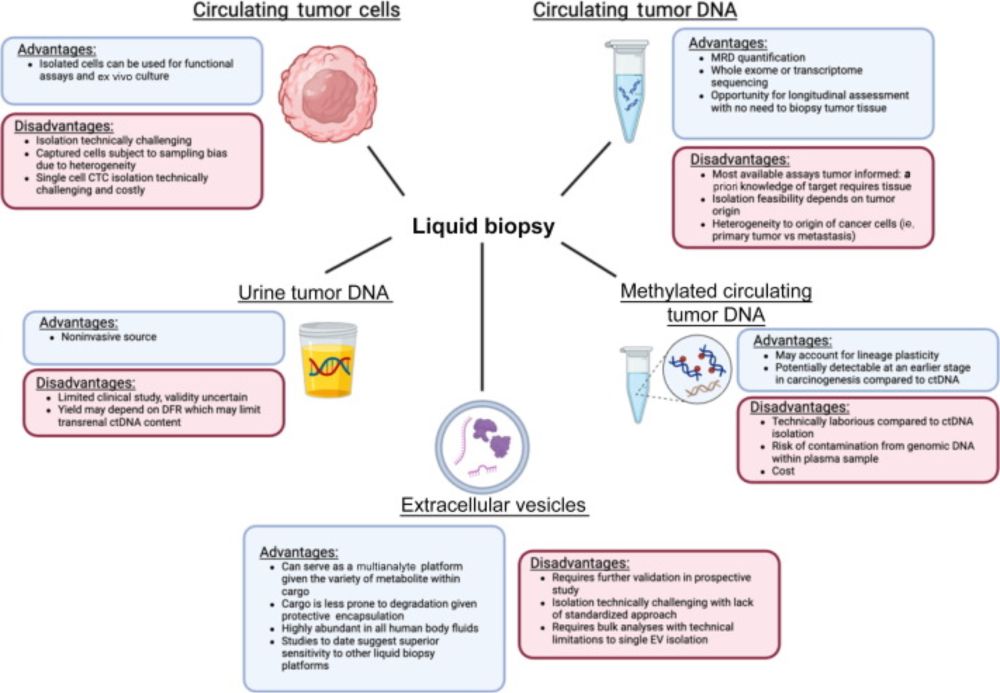
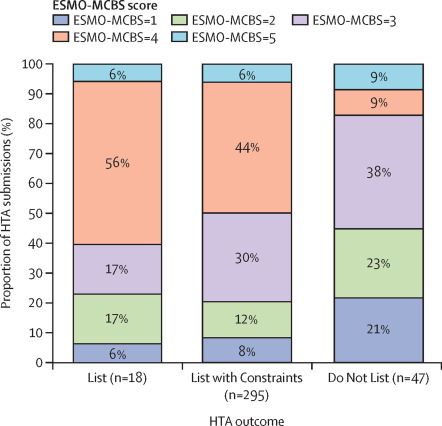
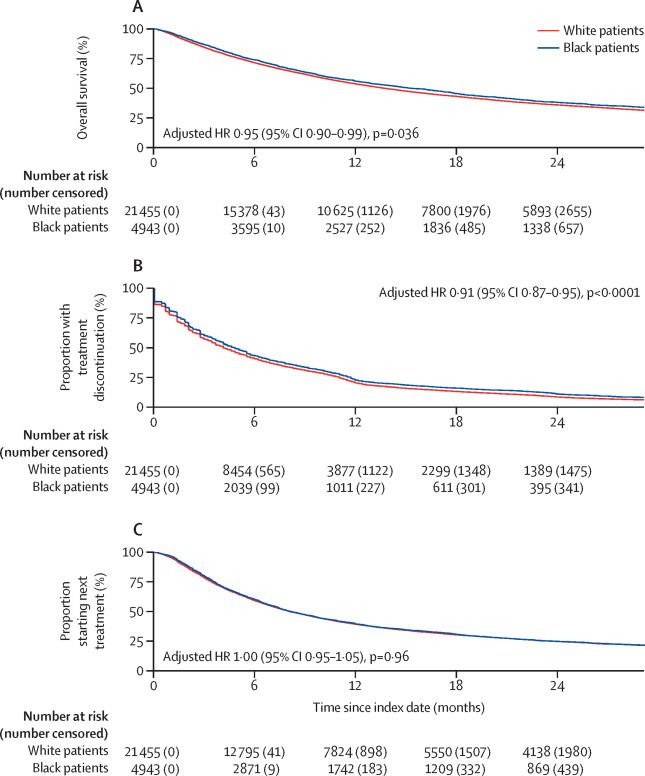
DEFINE_ME
Intensification Approaches and Treatment Sequencing in Metastatic Castration-resistant Prostate Cancer: A Systematic Review www.europeanurology.com/article/S030...
19.12.2024 10:25 — 👍 2 🔁 0 💬 0 📌 0
ASCO Publications
Darolutamide in Combination With Androgen-Deprivation Therapy in Patients With Metastatic Hormone-Sensitive Prostate Cancer From the Phase III ARANOTE Trial ascopubs.org/doi/10.1200/...
19.12.2024 10:23 — 👍 2 🔁 0 💬 0 📌 0

Excited to share the release of a new book on bladder cancer that I had the pleasure of editing together with Margaret Knowles, featuring contributions from multiple expert authors!
17.12.2024 19:53 — 👍 16 🔁 2 💬 3 📌 0
If you don’t allow prior taxanes, and your control arm also excludes chemo… then your trial results will have low external validity (if any), and patients treated in the trial may have been exposed to suboptimal systemic therapy.
16.12.2024 15:57 — 👍 1 🔁 0 💬 0 📌 0
85 people in here now, and growing - come and join us!
#Genitourinary #Oncology #GUonc #GUoncol #GUoncology #ProstateCancer #KidneyCancer #Urothelial #BladderCancer #PenileCancer #TesticularCancer #pcsm #kcsm #blcsm #tcsm #Medsky #Oncsky #Medtwitter
go.bsky.app/DtoXvaD
07.12.2024 21:29 — 👍 8 🔁 3 💬 1 📌 1

UK will have men's health strategy, government announces
The strategy may cover areas such as mental health, suicide prevention, heart disease and stroke.
🚹 UK government to announce #MensHealth strategy.
🚹 “We’re seeing mental ill health on the rise and the shocking fact that #suicide is the biggest killer for men under 50.”
🚹 “Preventable killers like #heartdisease and #prostatecancer are being caught far too late."
www.bbc.com/news/article...
29.11.2024 07:10 — 👍 5 🔁 4 💬 1 📌 1
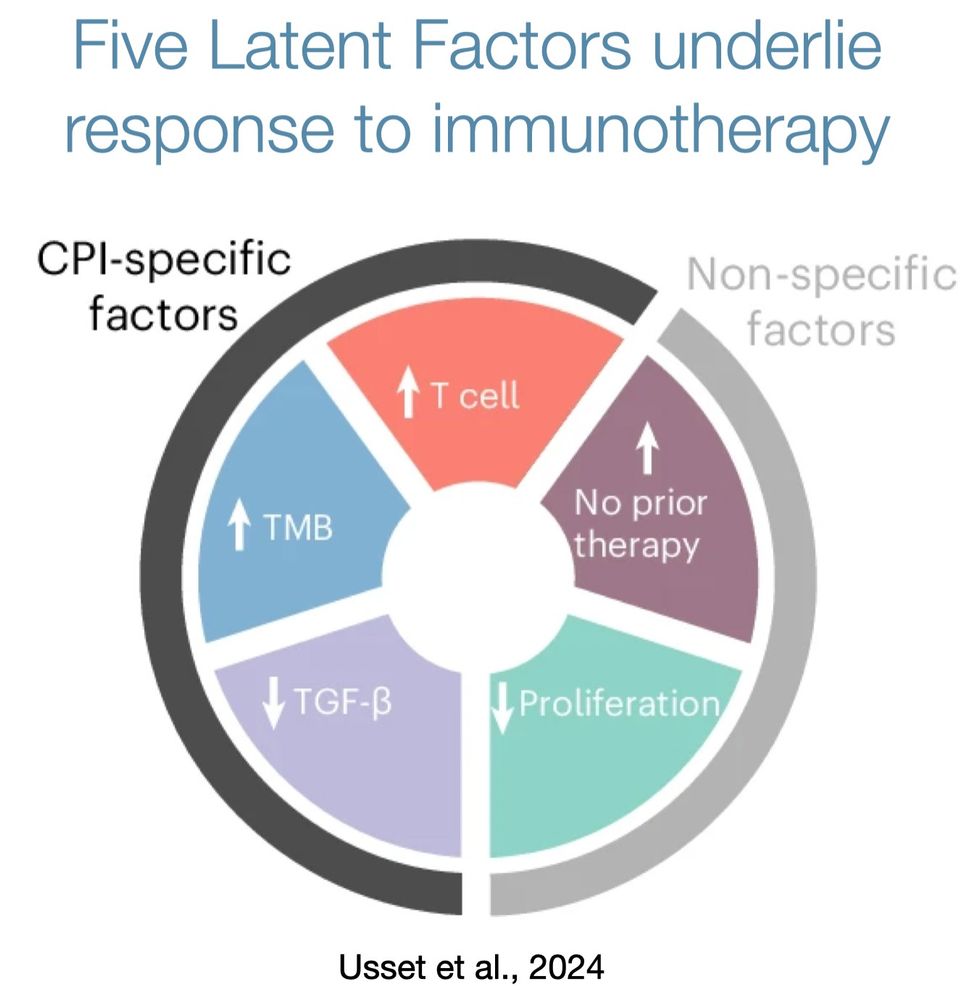
Only a subset of patients respond to immunotherapy
Why? Which ones?
We conducted an unbiased analysis of response biomarkers and found that hundreds of possible biomarkers collapse into five latent, orthogonal factors that underlie immunotherapy response.
www.nature.com/articles/s41...
27.11.2024 14:13 — 👍 132 🔁 47 💬 4 📌 5
Vigilamos al poder, cuidamos lo público.
Con periodismo, herramientas y acción.
civio.es
Apúntate a nuestro boletín: https://civio.es/boletin/
Assc. Director CCEP & Thoracic Oncologist @DanaFarberNews & @Harvardmed| Young Lung Cancer | Editor @Jamaonc | Co-founder @LatinasInMed | @Florez_Lab
Prostate and bladder cancer specialist, clinical trialist, passionate driver of innovation and change.
Medical Oncologist. Editor-in-chief: @ASCO Daily News; Presidential Endowed Chair, @HuntsmanCancer (NCI-CCC)
@UUtah , FCOI http://bit.ly/3A3u2KL More info: bit.ly/3JApRJd
Advancing oncology care through research, innovation, and multidisciplinary collaboration. Passionate about patient-centered solutions, PROs, HRQOL, and evidence-based practices, w a focus on #GUOnc, #GeriOnc & #PallOnc
#ASCO25 FCOI: http://bit.ly/344gKxq
A nonprofit raising awareness, promoting self-examination and helping survivors and caregivers navigate #testicularcancer and unite via community. #tscsm
Medical Oncologist focusing on urological cancer.
Fellow at Gustave Roussy
Main interests: #RCC #ProstateCancer
Professor of Urology, Berlin, Germany. Urologic Cancer Surgeon and urologic oncologist
Head of German Focal Treatment Experts
Head of https://logicuro.com
Oncóloga Medica. Tumores digestivos y neuroendocrinos.
Medical Oncologist. Gastrointestinal, neuroendocrine and endocrine tumors. #MedSky #OncSky #NETsSky
Formerly known as @alvarezperea in Twitter
Your daily dose of hope, inspiration, and information in the fight against cancer.
oncodaily.com
Oncologist, University of California San Francisco
ASCO President-Elect
Account of the American Society of Clinical Oncology & its affiliate the Association for Clinical Oncology.
The world’s leading professional organization representing more than 50,000 oncology professionals caring for people with cancer.
Professor and Associate Director of Translational Research, GU Medical Oncologist at the University of Wisconsin Carbone Cancer Center.
https://langlab.labs.wisc.edu/
#prostatecancer #cancerbiology #biomarkers
Consultant (clinician-scientist) in medical oncology with an interest in Lower GI malignancies including appendix tumours and peritoneal metastasis. #H12O #imas12 #rarecancers #translationalresearch #drugdevelopment
Clinical Oncologist, TPD, mother of 3, allotment enthusiast and occasional runner!
#radonc at University of Miami.
#Digitalhealth enjoyer. #digitaltransformation enthusiast.
Doesn’t have a podcast.
Stage IV kidney cancer (metastatic oncocytoma) | Software Developer | Founder of COA | Obsessed w/podcasts, medical science & nonfiction | Memoir - Too Young For Cancer | Views own
https://www.katiekickscancer.com