
Eric J. Chow et al. find that dexrazoxane given with doxorubicin in childhood cancer is linked to improved long-term cardiac function and lower risk of poor left ventricular function in adult survivors.
#JournalClub
ascopubs.org/doi/10.1200/...

Eric J. Chow et al. find that dexrazoxane given with doxorubicin in childhood cancer is linked to improved long-term cardiac function and lower risk of poor left ventricular function in adult survivors.
#JournalClub
ascopubs.org/doi/10.1200/...

Piotr et al. report that intravenous iron in a mouse cancer model increases tumor iron, promotes tumor growth, and blunts anti-tumor CD8⁺ T cell responses, reducing immunotherapy/chemo efficacy.
#JournalClub
www.frontiersin.org/journals/onc...

A shared DNA methylation drift occurs with aging and tumorgenesis in the colon. Age-related inflammation and reduced Wnt signaling, which dysregulate iron metabolism and impair TET activity, drive this epigenetic drift.
#JournalClub
www.nature.com/articles/s43...

Ferritin buffers intracellular iron and protects cells from iron toxicity. Yi et al. show that deleting the ferritin heavy chain (Fth1) impairs normal HSPC function but restricts AML progression and prolongs survival in mice.
#JournalClub
www.nature.com/articles/s41...




When Ning, Francesca & Marc were selected to speak
@senescel.org 2025, they gave it their all, refining every argument and minute.
Their talks put our metabolic bottleneck model of aging on the field’s radar. 👏
What do you think drives tissue aging, the cell or its environment? 🤔

Postdoc | VHIO, Barcelona
Immunotherapies succeed in only ~20% of cases.
Help us break resistance by engineering the right environment.
Join the Aging & Cancer Lab 🌍
👉 Apply: vhiovidaprogramme.eu/recruitment/ | mauslab.org

📖Culture dishes and in vivo studies are not our only experiments.
As our lab turns soon 3, we keep on testing how to grow a culture where ideas and people thrive.
For anyone curious about the science of teamwork, The Culture Code is a brilliant field guide.
📖 #LabReads

📃By integrating single-cell RNA sequencing datasets, Cong et al. characterize the evolution and interactome of hematopoietic niche from embryonic development to aging. #JournalClub
pubmed.ncbi.nlm.nih.gov/39698109/

Lung cancer reprograms the bone marrow to its advantage.
Hedge et al. ( @miriammerad.bsky.social lab)
show that tumors trigger an NRF2-driven stress response in myeloid precursors, which then suppress anti-tumor immunity. Blocking NRF2 boosts immunotherapy.
#JC
🔗 www.nature.com/articles/s41...




#CCESumSchool25 Day 2 🧬
• Lectures: Fundamental Research, Patient-centered Research, Proteomics
• Workshop: Patient Involvement
• First Poster Session!
@vhio.bsky.social @nkinl.bsky.social @nct-heidelberg.bsky.social @gustaveroussy.fr @ki.se @zwartlab.bsky.social @mauslab.bsky.social
Hear from Aistė Avižaitė, who joined us from Lithuania to explore aging & cancer and grow in translational oncology at @vhio.bsky.social.
▶️https://youtube.com/shorts/q0g6zdywe6s
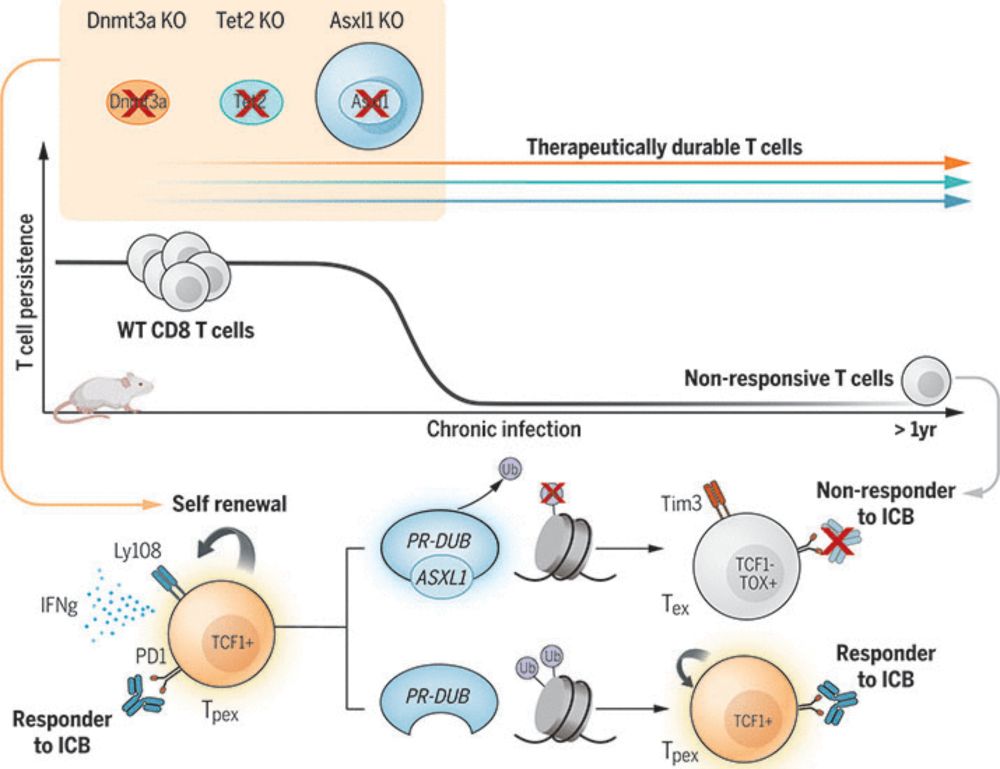
📰 In aging bone marrow, cancerous clones thrive by adapting through mutations. Kang et al. show that giving immune cells the same mutations tilts the balance back—helping them resist exhaustion and fight cancer in this hostile niche. #JournalClub
🔗 www.science.org/doi/10.1126/...
📰Aging disrupts metabolic supply chains, constraining cell renewal. But how do we detect these shortages? In >5000 patients, Tapio et al. show that low ferritin is the best predictor for bone marrow iron. A study with broad implications. hashtag #JournalClub
🔗 ashpublications.org/bloodadvance...
📑Why don’t senescent cells fix their DNA? Daigh et al. (Meyer lab) report that uncoupling mTORC1 from E2F sustains transcription-linked DNA damage in senescence.
#JournalClub
www.nature.com/articles/s41...

Why do TET2-mutant preleukemic cells outcompete healthy HSCs? Yang et al. show that these cells upregulate MPL (thrombopoietin receptor, TPO-R) signaling, giving a clonal edge; inhibiting this pathway reduces it. #JournalClub
www.nature.com/articles/s41...

Why do ring sideroblasts arise in MDS? Often via SF3B1-driven mitochondrial iron buildup. Inokura et al. tested TET2’s role: Tet2-deficient mice show systemic iron overload, and anemia. No classical RS, but intriguing parallels.
#JournalClub
www.exphem.org/article/S030...

🌱We are delighted to welcome Gloria Ariño from Universidad Europea de Madrid as our third summer intern!🌟
Gloria will explore how aging creates metabolic bottlenecks in the colon and how these shape tumorigenesis.
With Gloria, Laura & Aiste, our summer intern dream team is complete!💪

📑Immunotherapy responses differ markedly between MMR-proficient and -deficient colorectal cancers. A scRNA-seq study of 62 patients by @pelkalab.bsky.social et al. (Hacohen lab) reveals key immune–tumor communication differences that may explain this.
#JournalClub
www.cell.com/cell/fulltex...

🎯 The Aging and Cancer Lab is not afraid of tough battles — in science or laser tag.
What connects aging and cancer? Can we stop it?
We team up and take aim together at the route causes.. 🔬💥

🌱Excited to welcome Aistė Avižaitė from Lithuania’s
@VU_LT
to the lab this summer!
She’ll be studying how chemotherapy alters metabolism in healthy tissues and how this may drive accelerated aging in childhood cancer survivors.
#Aging #Cancer #SummerIntern
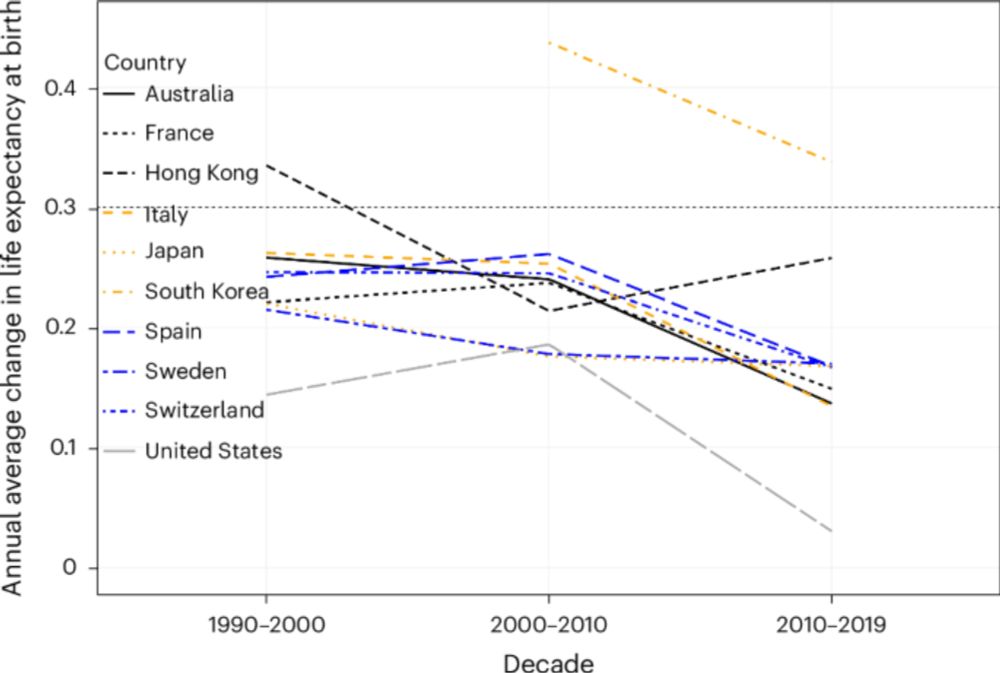
200 years ago in Barcelona, your 40s meant preparing for death. Today, you might have 40 more years ahead.
But as @sjayolshansky et al. show, most gains came from reducing early death. Further progress depends on tackling aging and its consequences.
#JC
www.nature.com/articles/s43...

🌱 Welcome aboard, Laura!
Joining us this summer from @URV_universitat, Laura Boró García will be exploring how aging and chemotherapy disrupt metabolism and epigenetics in the bone marrow. We’re thrilled to have her with us! 🧬🧪
#Aging #Cancer #SummerIntern
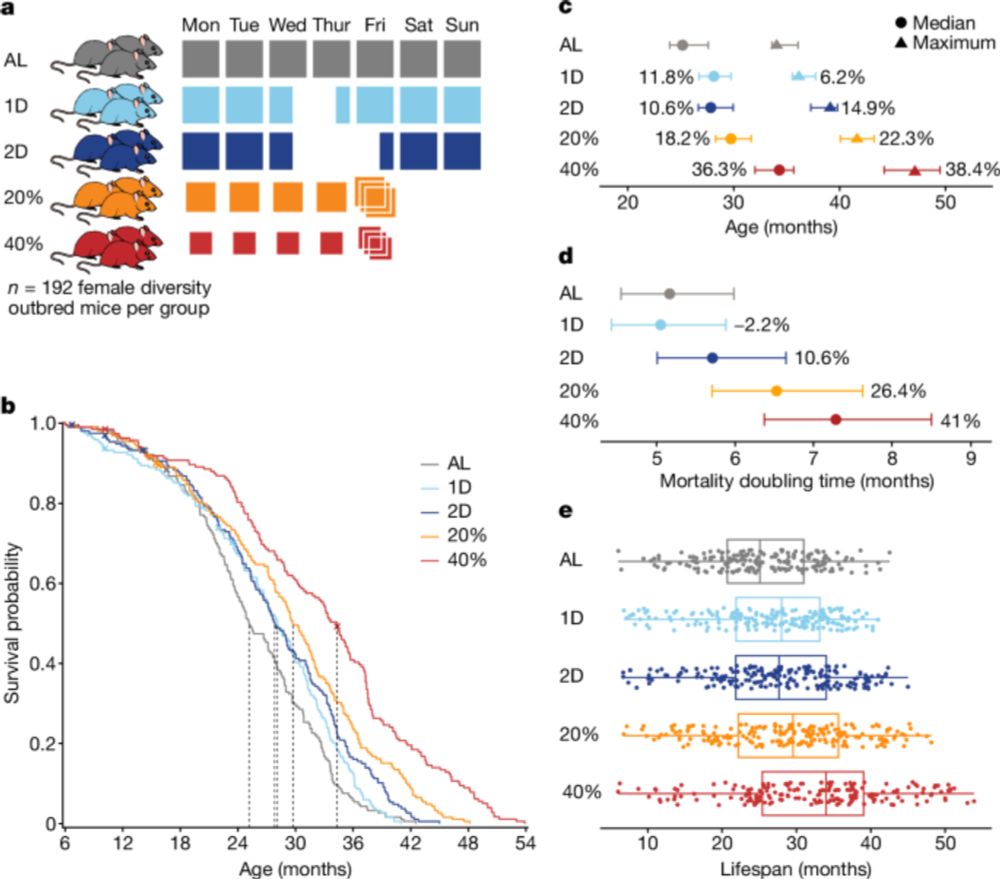
Longer lives in laboratory conditions may compromise survival in the real world. In a large study on genetically diverse mice, Di Francesco et al. in Jackson Labs show that lifespan extension under caloric restriction doesn’t always improve health.
#JournalClub
www.nature.com/articles/s41...

Longer lives in laboratory conditions may compromise survival in the real world. In a large study on genetically diverse mice, @DiFrancescoA et al. in @jacksonlab show that lifespan extension under caloric restriction doesn’t always improve health.
#JournalClub
www.nature.com/articles/s41...
In a preprint, Han et al. from @yibinkang’s lab report that cancer cells in bone metastases hijack iron from bone marrow macrophages that normally support red blood cell production — linking anemia to metastatic progression.
#JournalClub
www.biorxiv.org/content/10.1...

What makes a bag of cells become a complex, intelligent being—and what breaks those rules to cause aging and system breakdown?
Donella Meadows’ Thinking in Systems offers a toolkit to understand biology, society, and the economy in their native language: systems.
#LabReads 📚
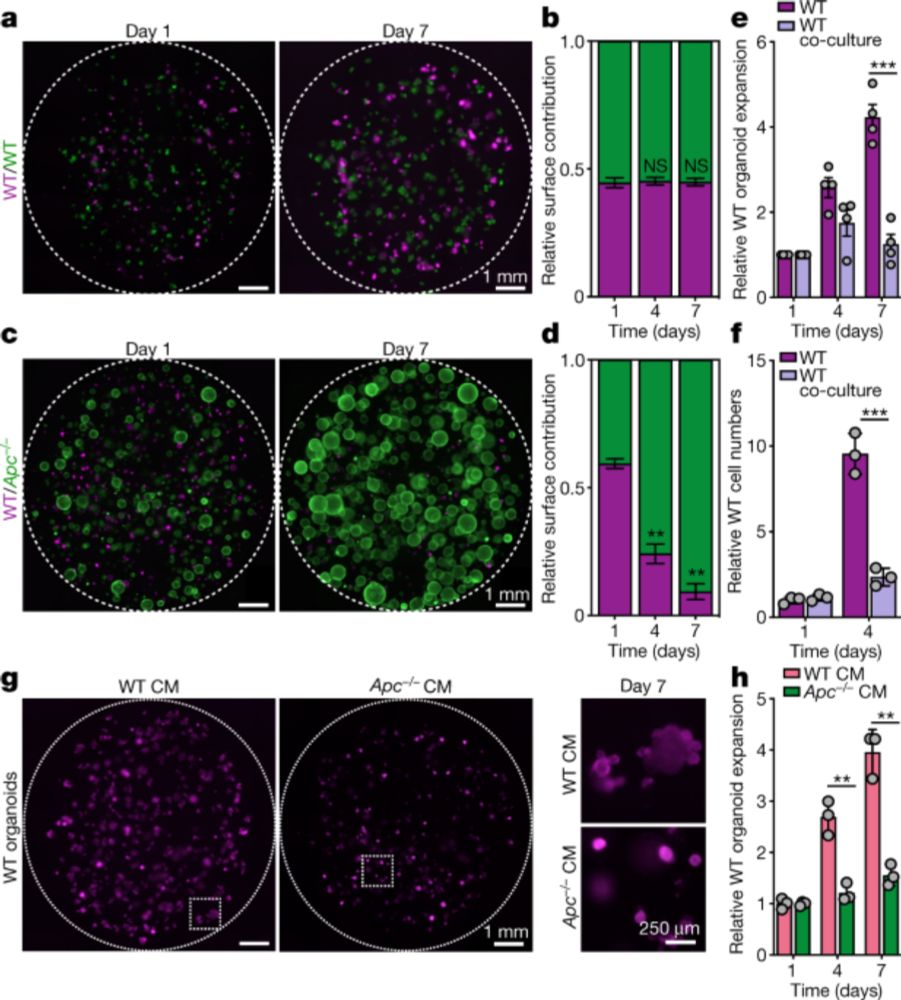
Intestinal stem cells (ISCs) with APC mutations outcompete wild-type ISCs by suppressing Wnt signaling in their neighbors - driving colorectal tumor formation.
Exciting work from Vermeulen lab!
Presented by Marc Guasch @JournalClub
www.nature.com/articles/s41...
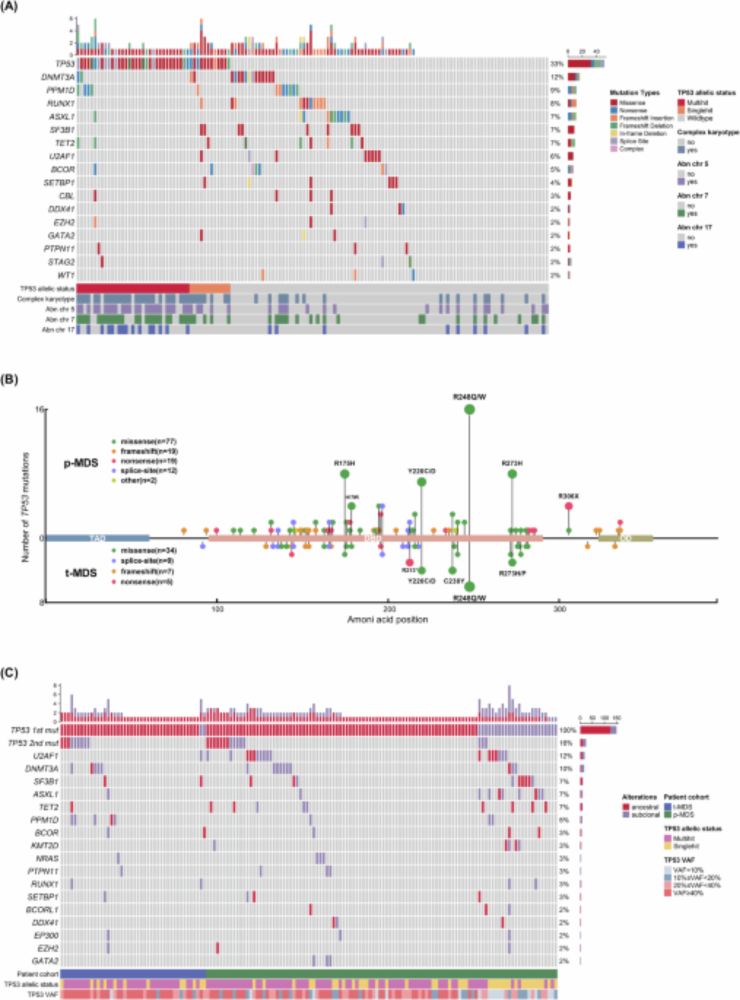
We are all born with blood cells that could turn into cancer. But aging and chemo don’t just damage cells—they differently pick the bad ones. Chemo favors TP53-mutant clones giving rise to therapy-related MDS.
#JournalClub Ning Huang
www.nature.com/articles/s41...
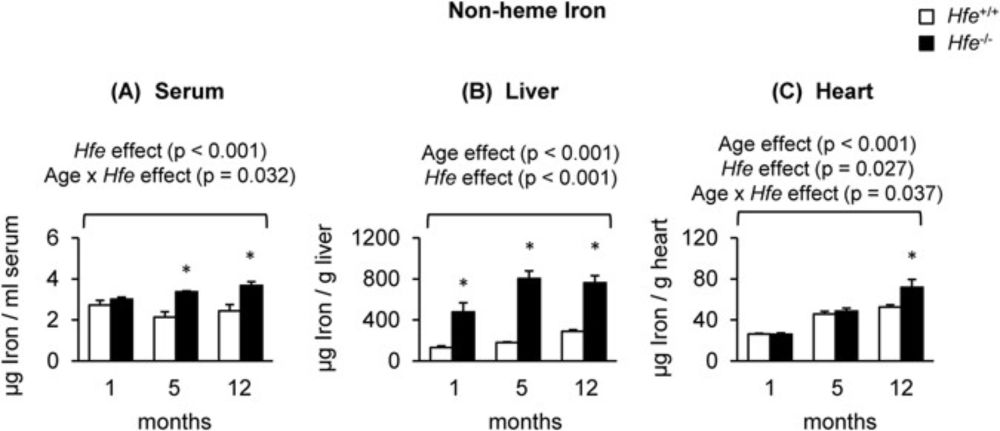
Hereditary hemochromatosis related iron overload accelerates age-related cardiac hypertrophy in a mouse model.
Francesca Cogo #JournalClub
www.nature.com/articles/s41...

Increasing number of young individuals are being diagnosed with colorectal cancer. This week, we hosted medical oncologist Iosune Baraibar, who shared what she at
@vhio.bsky.social, and @danafarber.bsky.social collaborators are doing to understand and act on this epidemic. Honored to work at VHIO.