A big thank you to everyone involved for all the hard work!
18.01.2026 21:45 — 👍 1 🔁 0 💬 0 📌 0Ribbe Hu Labs
@ribbehulab.bsky.social
Metalloprotein assembly and catalysis; bioinorganic chemistry; structural biology; spectroscopy; microbiology
@ribbehulab.bsky.social
Metalloprotein assembly and catalysis; bioinorganic chemistry; structural biology; spectroscopy; microbiology
A big thank you to everyone involved for all the hard work!
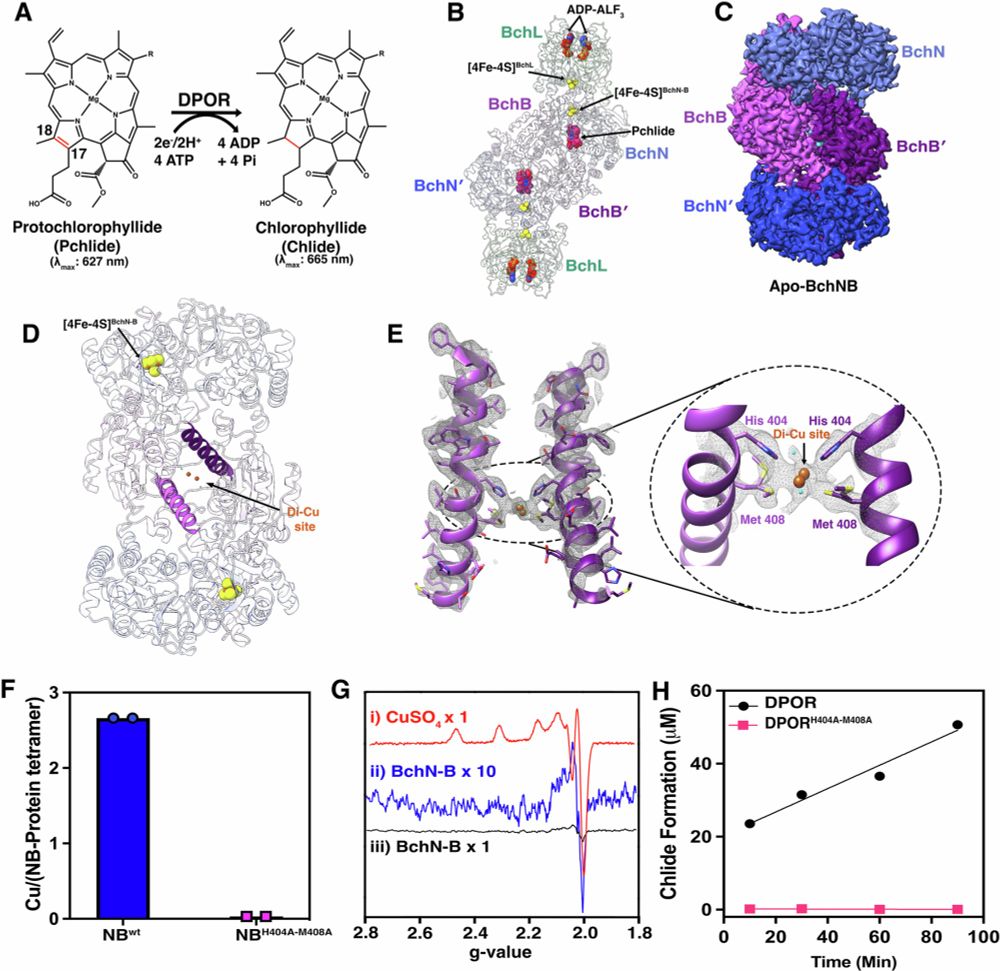
18.01.2026 21:45 — 👍 1 🔁 0 💬 0 📌 0Many thanks, Ruben, for the opportunity to showcase our work, “Crossing Enzymatic Boundaries by Coupling BchNB with the Nitrogenase Cofactor Precursor,” in ChemBioChem!
@rubenragg.bsky.social
Who would have thought? We successfully attached the nitrogenase L-cluster to DPOR.
@ribbehulab.bsky.social @ucibiosci.bsky.social
chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/...
Our latest work on the nitrogenase-like methylthio-alkane reductase, which specifically reduces reduces carbon-sulfide bonds is now out @natcatal.nature.com: doi.org/10.1038/s419.... We find for the first time large #nitrogenase metalloclusters (P- and L-cluster) outside nitrogenases.
23.10.2025 10:09 — 👍 49 🔁 20 💬 4 📌 1
Our home department of Molecular Biology & Biochemistry at UC Irvine is looking to hire a new tenure-track assistant professor in the broad area of structural biology. Come be our colleague! recruit.ap.uci.edu/JPF09887
Please apply and/or share this post.

Congratulations to Bryan Neumann from our collaborator Shane Gonen’s lab on receiving the Barbara K. Burgess Postdoctoral Fellowship Award — a well-deserved honor!
@gonenshane.bsky.social @ribbehulab.bsky.social @ucibiosci.bsky.social
Thanks for your kind words Joe!
06.05.2025 19:09 — 👍 0 🔁 0 💬 0 📌 0Thanks for your kind words Sven!
06.05.2025 19:06 — 👍 1 🔁 0 💬 0 📌 0Thanks for your kind words!
06.05.2025 19:05 — 👍 1 🔁 0 💬 0 📌 0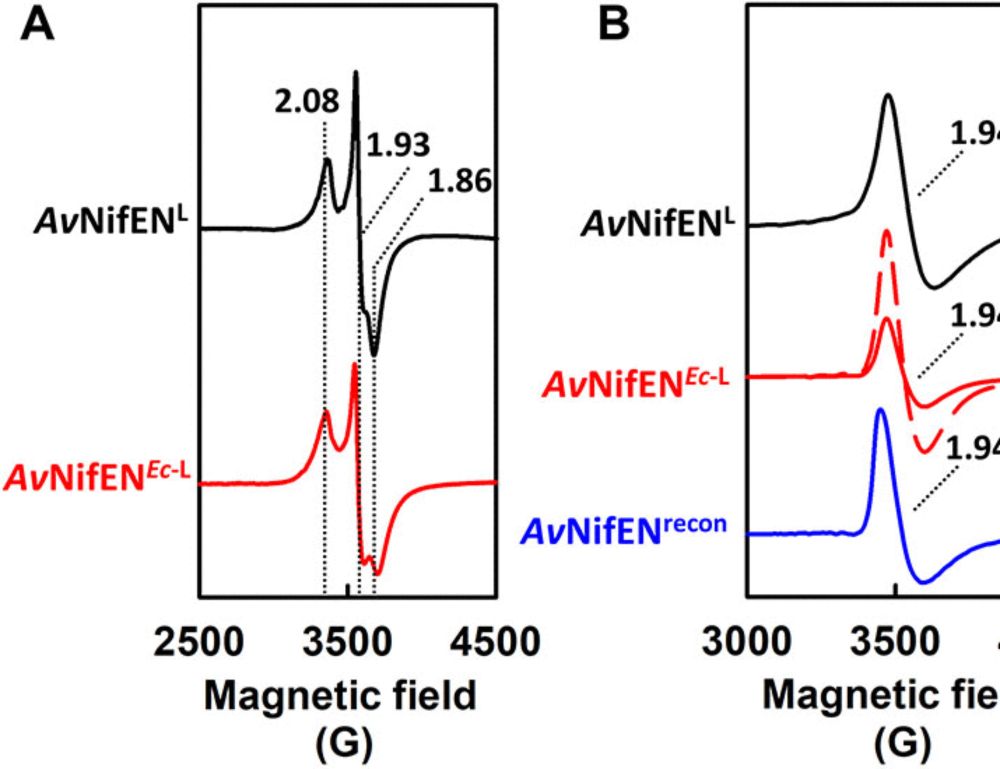
Our second paper that came online within a week 😊! Thanks everyone for their hard work!
@ribbehulab.bsky.social @ucibiosci.bsky.social
Heterologous synthesis of a simplified nitrogenase analog in Escherichia coli | Science Advances www.science.org/doi/10.1126/...
Our story detailing how asymmetry in nitrogenase-like proteins regulate electron transfer reactions is finally out! rdcu.be/ejaeK Congratulations to postdoc @rajnandani.bsky.social and fantastic collaborators. Thanks to funding from the Department of Energy and the NIH.
24.04.2025 14:11 — 👍 19 🔁 6 💬 1 📌 3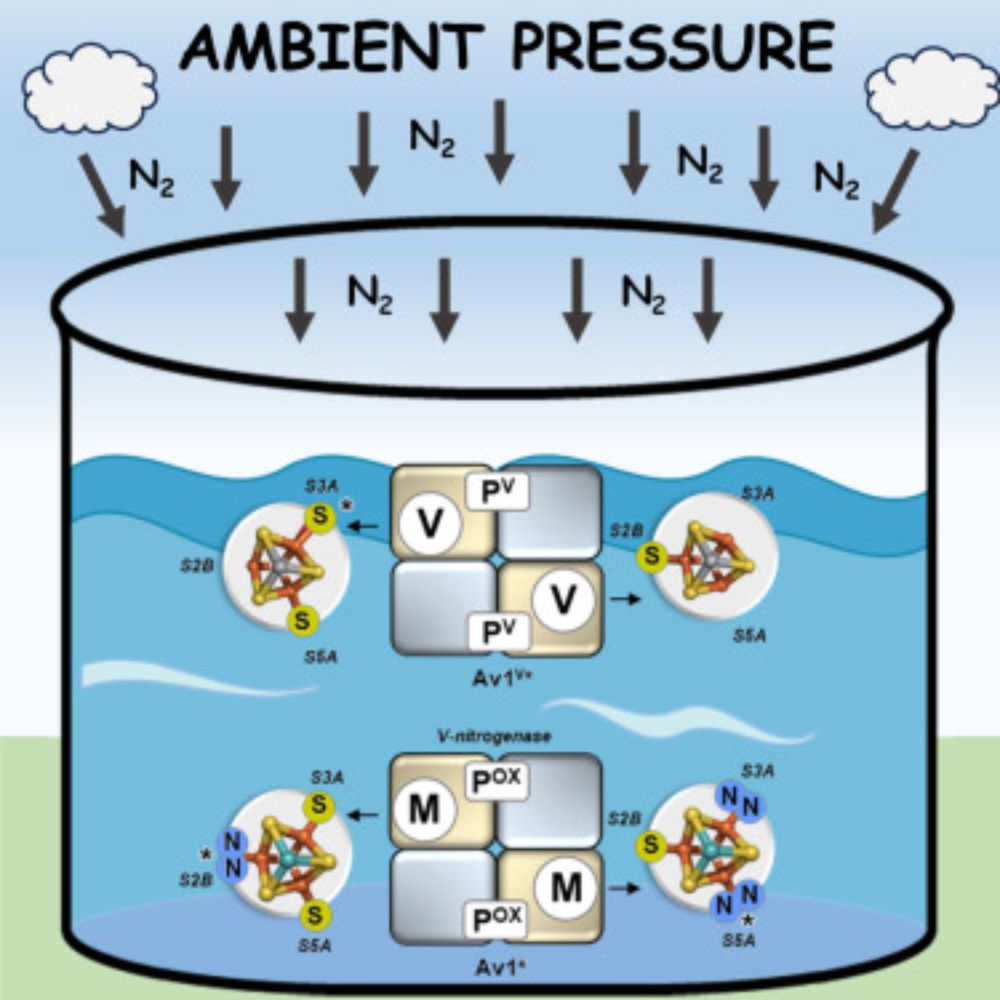
Excited to share our latest work highlighting the crucial role of belt-sulfur mobilization in nitrogenase catalysis. A big thank you to everyone who contributed to this effort!
@cp-chemcatalysis.bsky.social @ucibiosci.bsky.social
www.cell.com/chem-catalys...
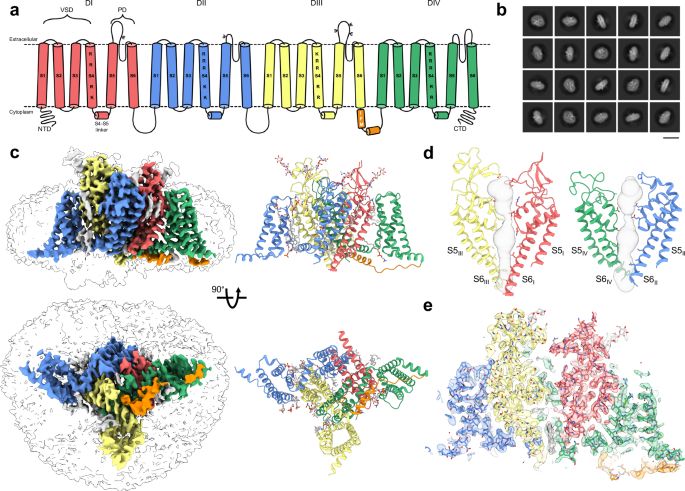
Our #CryoEM study on the binding of a Tarantula toxin to a full-length human voltage-gated sodium channel has been published. Very proud of the awesome people in my lab 🥳🥂
www.nature.com/articles/s41...