
Not too often you see HRQoL data in drug commercials. Via Ari Gnanasakthy youtu.be/VDFF6MCFzKM?...
08.04.2025 12:35 — 👍 2 🔁 0 💬 0 📌 0@refiningvalue.bsky.social
Health economist writing about HEOR/market access, drug development, biopharma Http://refiningvalue.substack.com

Not too often you see HRQoL data in drug commercials. Via Ari Gnanasakthy youtu.be/VDFF6MCFzKM?...
08.04.2025 12:35 — 👍 2 🔁 0 💬 0 📌 0Oh completely agree on lifestyle. Not sure our HHS secretary realizes that or cares though
03.04.2025 13:24 — 👍 1 🔁 0 💬 0 📌 0I would be surprised given RFK's public skepticism on GLP-1s, suggesting "lifestyle changes" should be the focus
03.04.2025 13:15 — 👍 1 🔁 0 💬 1 📌 0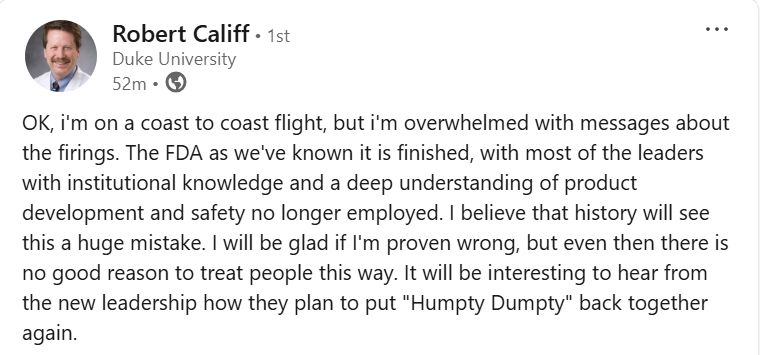
Former FDA Commissioner:
01.04.2025 12:59 — 👍 1042 🔁 372 💬 27 📌 24
Nice paper outlining how RWE can be applied in IRA evaluation of drugs academic.oup.com/healthaffair...
26.03.2025 16:24 — 👍 2 🔁 0 💬 0 📌 0
Glad to see GLP-1s continue to push expanded direct offerings: www.prnewswire.com/news-release...
06.03.2025 13:03 — 👍 1 🔁 0 💬 0 📌 0Reports and documentation are a low-hanging fruit for biopharma and can help reduce OpEx, especially that which is currently outsourced to vendors. CSRs, other study reports, payer dossiers, briefing book docs etc etc. Big potential for short to medium-term savings
05.03.2025 18:54 — 👍 5 🔁 3 💬 0 📌 0Short-termism driven by personal timelines rule the day. Greater purpose be damned.
Would a Vagelos or Termeer have done the same? I would think not, and hopefully history judges the two archetypes accordingly.
Was really expecting more of these people.
19.02.2025 17:14 — 👍 17 🔁 4 💬 3 📌 1I am grieving for the many talented & dedicated FDA employees who have been mistreated & those left to do the work of protecting public health. Also for those who will be harmed by this among patients and the public. Those who are complicit in their silence will be judged harshly by history.
17.02.2025 03:34 — 👍 96 🔁 30 💬 1 📌 4I am going to be so pissed if--after everything--I do, in fact, die of dysentery.
13.02.2025 21:33 — 👍 4092 🔁 847 💬 86 📌 85Also, while the FDA of the next 4 yrs may not care, the FDA of the future may care once more. And how many pivotal trials being started now will have a PDUFA date within 4 years? Probably not the vast majority
13.02.2025 23:07 — 👍 2 🔁 1 💬 0 📌 0Even from a development economics perspective. Reaching underserved populations, those away from academic centers, etc much needed to help trial recruitment and trial timelines.
Hopefully this is just trying to fly with the political wind without really changing behavior.

Defend the NIH. timmermanreport.com/2025/02/defe...
13.02.2025 17:04 — 👍 9 🔁 8 💬 0 📌 1
Biopharma companies and CEOs are keeping their heads down at their own peril. They should speak up about what’s happening to the NIH and other science agencies before it’s too late.
Silence gives consent. And no one should consent to this.
Ah mightve just assumed George was same as Len. Still feel like with the science emphasis + harder to remove them they're most likely
12.02.2025 11:28 — 👍 0 🔁 0 💬 1 📌 0Other issue here is unlike tech no one is irreplaceable (like Zuck) because no one owns enough voting rights, at least amongst the bigger players -> riskier to act. Len/George at Regeneron most likely to say something imo (especially given general outspokeness and strong Dem support historically)
12.02.2025 00:06 — 👍 1 🔁 1 💬 1 📌 0To some extent same for board members and larger investors. Even moreso w backdrop of hoping IRA implementation isn't as aggressive. But I suspect it could catch up to the industry, much as years of ignoring drug pricing issues has. But for those in charge today it probably won't be "their problem".
12.02.2025 00:06 — 👍 1 🔁 0 💬 1 📌 0Of course not reflective of many (most?) of us working in the industry.
Unfortunately, anything at NIH today that will lead to a new drug is way too long a time horizon for leadership to worry about personally (chances are they'll be long gone by the time it comes to fruition).
![TOTE ASTRICT COLOR
DOCTORS FOR AMERICA,
Plaintiff,
Civil Action No. 25-322 (JDB)
OFFICE OF PERSONNEL MANAGEMENT et al.,
Defendants.
ORDER
Upon consideration of [6] Plaintiff's motion for a temporary restraining order, [8]
Plaintiff's supplemental declarations, [9] Defendants' opposition, [10] Plaintiff's reply, the
hearing on February 10, 2025, and the entire record herein, and for the reasons stated in the
accompanying Memorandum Opinion, it is hereby ORDERED that
1. Defendants Department of Health and Human Services, Center for Disease Control, and Food
and Drug Administration (hereinafter "defendants") shall, by not later than 11:59 pm on
February 11, 2025, restore to their versions as of January 30, 2025, each webpage and dataset
identified by Plaintiff on pages 6-12 of its Memorandum of Law in Support of the Motion for
a Restraining Order [ECF No. 6-1];
2. Defendants shall, in consultation with Plaintiff, identify any other resources that DF A members
rely on to provide medical care and that defendants removed or substantially modified on or
after January 29, 2025, without adequate notice or reasoned explanation; and defendants shall,
by February 14, 2025, restore those resources to their versions as of January 30, 2025;](https://cdn.bsky.app/img/feed_thumbnail/plain/did:plc:36eqtmzysqf7wsslczw4uxcd/bafkreifoaihlyxzbiko32lmralzpicfxnzwfoxp3ki5w4wniiihonzxira@jpeg)
TOTE ASTRICT COLOR DOCTORS FOR AMERICA, Plaintiff, Civil Action No. 25-322 (JDB) OFFICE OF PERSONNEL MANAGEMENT et al., Defendants. ORDER Upon consideration of [6] Plaintiff's motion for a temporary restraining order, [8] Plaintiff's supplemental declarations, [9] Defendants' opposition, [10] Plaintiff's reply, the hearing on February 10, 2025, and the entire record herein, and for the reasons stated in the accompanying Memorandum Opinion, it is hereby ORDERED that 1. Defendants Department of Health and Human Services, Center for Disease Control, and Food and Drug Administration (hereinafter "defendants") shall, by not later than 11:59 pm on February 11, 2025, restore to their versions as of January 30, 2025, each webpage and dataset identified by Plaintiff on pages 6-12 of its Memorandum of Law in Support of the Motion for a Restraining Order [ECF No. 6-1]; 2. Defendants shall, in consultation with Plaintiff, identify any other resources that DF A members rely on to provide medical care and that defendants removed or substantially modified on or after January 29, 2025, without adequate notice or reasoned explanation; and defendants shall, by February 14, 2025, restore those resources to their versions as of January 30, 2025;
BREAKING: In response to doctors' lawsuit, Judge John Bates, a George W. Bush appointee, orders CDC, NIH, and FDA to put back up websites and datasets cited by the doctors in their lawsuit as having been relied upon and pulled down without notice. storage.courtlistener.com/recap/gov.us...
11.02.2025 17:15 — 👍 28305 🔁 6780 💬 296 📌 284Best proxies on non-profit behavior in these circumstances may be in healthcare space w number of large non-profit hospital and insurer (eg blue cross blue shield plans) deals over the years, albeit none of this size. Spoiler is they behave pretty much like their for-profit counterparts
11.02.2025 17:38 — 👍 0 🔁 0 💬 0 📌 0
This is the most relevant article to NIH and research cuts I’ve seen.
Imagine if this was today , how many people would be saying “Why are we studying Gila Monsters and their impact on diabetes ? That’s wasted money !”
globalnews.ca/news/9793403...
Pharma bet that Trump>Biden may be tested sooner than I thought w the news of FDA cuts. Especially if review timelines / meeting timelines start getting impacted
07.02.2025 00:09 — 👍 1 🔁 0 💬 0 📌 0Coupling modeling of price change w contractual agreement on price drop at date x (a la Peter Kolchinsky's contractual genericization) could be a win-win here and reduce some uncertainties.
Re competitive landscape maybe something like NICE's TA-level CEA in RCC but that has it's own issues
Some interesting parallels re Deepseek: US-based LLMs and the bio explosion in China:US biotech
Still feel it'll be net positive for the industries and consumers at large in both cases
If we assume they kept the highest NPV internal assets going then you can compare the top internal v top external assets from that time (not like he killed everything at Sanofi). I guess you can argue forecasting sucks and w larger net it mightve been different but hard to say.
25.01.2025 11:28 — 👍 0 🔁 0 💬 1 📌 0Regeneron seems to be the clearest example to me. Maybe Genmab? Vertex?
25.01.2025 11:16 — 👍 1 🔁 0 💬 0 📌 0I shouldn't try to apply logic here and apologies for the rare political post but if NIH is developing all of the drugs then why are we freezing all of the funding again?
24.01.2025 19:24 — 👍 3 🔁 1 💬 0 📌 0Not sure if it's a great example against external. Genzyme and extension of Regeneron deal under Viehbacher are still a huge part of sales and growth for the co today vs the internal assets from the time (eg Toujeo) which haven't done nearly as well
24.01.2025 19:17 — 👍 1 🔁 0 💬 1 📌 0Of course the elephant in the room is still that clinical development will remain super expensive, largely driven by Western development infrastructure costs. No China advantage there from what I can tell. Although it is nice to see alternative models from Beigene etc v the traditional CRO model
22.01.2025 14:24 — 👍 0 🔁 0 💬 0 📌 0