New @chemrxiv.org, a collaboration with @honggen-wang.bsky.social and group alum, now asst prof. Myojeong Kim: the first true catalytic interception of isodiazene-generated primary-amine-derived radicals. The key is an N-F reagent that enables productive chain carrying!
doi.org/10.26434/che...
21.10.2025 01:13 — 👍 15 🔁 4 💬 1 📌 0

Early Career Research (ECR) Group Leader position (m/f/d) with the thematic focus on “Catalysis” within the Cluster of Excellence RESOLV
Please check this announcement for an early-career group leader, hosted jointly by our cluster of excellence RESOLV and the MPI Kofo. The thematic focus should be on catalysis: jobs.ruhr-uni-bochum.de/jobposting/4...
02.10.2025 13:54 — 👍 12 🔁 12 💬 0 📌 0


🚨 PhD Opportunity! 🚨
Join our group in Bioinorganic Chemistry to work on the synthesis & biological evaluation of metal complexes for anticancer therapy.
jobs.ruhr-uni-bochum.de/jobposting/8...
02.10.2025 10:16 — 👍 13 🔁 9 💬 0 📌 0
Thanks Tobi!
04.09.2025 19:14 — 👍 0 🔁 0 💬 0 📌 0

I’m proud to share that my group has received a Starting Grant from @erc.europa.eu, which will enable us to explore new organophotomediated reactions, and deeply grateful to my group and the many colleagues who helped with the application.
04.09.2025 13:51 — 👍 20 🔁 1 💬 2 📌 0
🚀 Join Us!
We have an exciting opportunity to join our research group!
This fully funded PhD position is part of the IPCat training programme.
Feel free to reach out if you have any questions or need more information.
👉 Apply now: www.findaphd.com/phds/project...
#PhD #compchem
18.03.2025 12:51 — 👍 20 🔁 13 💬 0 📌 1

Multiprobe Photoproximity Labeling of the EGFR Interactome in Glioblastoma Using Red-Light
Photocatalytic proximity labeling has emerged as a valuable technique for studying interactions between biomolecules in a cellular context, providing precise spatiotemporal control over protein labeling. One significant advantage of these methods is their modularity, allowing the use of a single photocatalyst with different reactive probes to expand interactome coverage and capture diverse protein interactions. Despite these advances, fewer methods have been developed using red-light excitation, limiting the use of photoproximity labeling in more complex media such as tissues and animal models. Herein, we develop a platform for proximity labeling under red-light excitation, utilizing a single catalyst and two distinct probe types. We first design a carbene based labeling system that utilizes sulfonium diazo probes. This system is successfully applied on A549 cells to capture the interactome of epidermal growth factor receptor (EGFR) using a Cetuximab-Chlorin e6 conjugate. Benchmarking against established techniques indicates that this approach performs comparably to leading carbene-based proximity labeling methods. Next, we leverage the strong singlet oxygen generation (SOG) ability of Chlorin e6 to establish an alternative labeling system using aniline and hydrazide probes. EGFR directed chemoproteomics experiments reveal significant overlap with the carbene system, with the carbene approach capturing a subset of interactions identified by the SOG system. Finally, we deploy our approach for the characterization of EGFR in resected human glioblastoma (GBM) tissue samples removed from distinct locations in the same tumor, representing the tumor’s infiltrating edge and its viable center, identifying several GBM specific interacting proteins that may serve as a launch point for future therapeutic campaigns.
Our first paper is out today in @jamchemsoc.bsky.social!
This is the beginning of a long journey for us, to study heterogeneity in GBM patient samples.
First step was to figure out some chemistry to enable us to look at this challenging problem.
pubs.acs.org/doi/10.1021/...
07.03.2025 19:36 — 👍 6 🔁 3 💬 1 📌 1
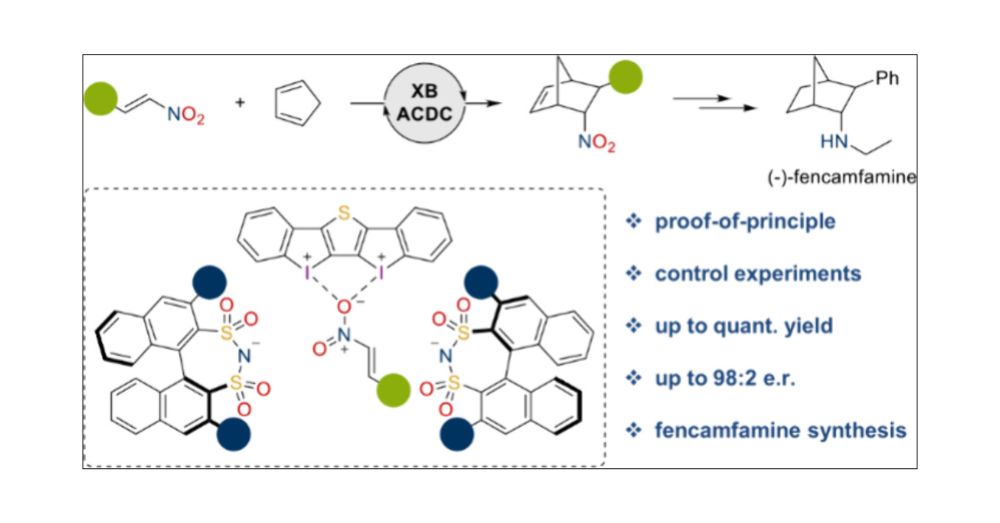
Asymmetric Counteranion-Directed Halogen Bonding Catalysis
Halogen bonding has been established as a promising tool in organocatalysis. Asymmetric processes are nevertheless scarce, and their applications are limited to a few studies applying chiral halogen b...
The combined power of halogen bonding and asymmetric counteranion-directed catalysis = XB-ACDC: pubs.acs.org/doi/10.1021/.... Thanks to @mertenlab.bsky.social for structure elucidation and to the List group for this beautiful cooperation! A perfect #RESOLV project! @solvationsci.bsky.social
03.03.2025 19:40 — 👍 13 🔁 5 💬 0 📌 2
We start our first post on Bluesky with a firework! Very proud of a brilliant team to publish our work on C(sp3)-atom transfer @science.org. www.science.org/doi/10.1126/... It has been a very exciting journey. Thanks @erc.europa.eu
20.02.2025 19:42 — 👍 121 🔁 28 💬 6 📌 4
Thanks Nick!
04.12.2024 18:08 — 👍 1 🔁 0 💬 1 📌 0

Our study on the partial reduction of arenes to cyclohexenes is now online in J. Am. Chem. Soc. Congrats to Kirti and Asad! pubs.acs.org/doi/full/10....
04.12.2024 14:46 — 👍 9 🔁 3 💬 0 📌 0

I am beyond excited to announce that I will be joining the Department of Pharmaceutical Sciences at the University Vienna (@univie.ac.at) as a Tenure Track Professor in March 2025! Thanks to the support of the LMU, I will also continue to work as a group leader in Munich for the foreseeable future!
01.12.2024 13:41 — 👍 90 🔁 3 💬 15 📌 0
We are a group of young organic chemists at the Department of Organic Chemistry, IISc Bangalore, India
https://atbiju.in/
Research professor at Institut de Ciència de Materials de Barcelona (CSIC), sustainable materials, chirality and supramolecular chemistry. Group and other stuff, own views here.
https://orcid.org/0000-0003-1674-8462
Junior Research Group Leader (Theoretical Chemistry) at TU Berlin.
Royal Society University Research Fellow at the University of St Andrews
https://craigpj2.wixsite.com/johnstonlab
Computational Organic Chemist
@University of Manchester #CompChem 💻 ⚛️ #Catalysis
🇪🇸 → 🇬🇧 | Mum of 3 boys 🤩
Cyclist 🚴♀️ | Yogi 🧘♀️ | Amateur cook
Rock music fan 🎸
🔗 https://trujilloresearchgroup.com/
PhD student in the List-Lab (Max-Planck für Kohlenforschung) | Previously: MedChem Intern @Roche | MRes @University of Oxford (Ed Anderson-, Duarte-Group) | KIT Karlsruhe (BSc)
Scientific Editor for Chemical Science, published by the RSC, and small-time journalist for Chemistry World. #ChemSky #ChemSci
Gaming, ballroom dancing, rollerskating, piano, and D&D - I’m acceptably mediocre at all of them! #Switch2
(he/him ♓️🏳️🌈)
Junior PI at #FUBerlin in Organic Chemistry. More info: https://www.bcp.fu-berlin.de/en/chemie/chemie/forschung/OrgChem/haut/index.html
Postdoc Morandi Lab ETH Zurich | PhD List Lab MPI für Kohlenforschung
Senior Business Development Manager at Thieme Chemistry for chemical databases. All views are my own.
Science of Synthesis editor at Thieme. Chemist. Runner, supporter of The Posh, and a bit of darts. Dad of two at the Ostsee coast.
Synthetic Organic Chemistry at TU Dortmund:
www.ccb.tu-dortmund.de/hansmann
Food scientist, Professor @ University of Hohenheim, Germany. Formerly Erlangen & Bristol. Lipids and lipid oxidation in food and archaeology, analytical chemistry. ORCID: 0000-0002-4311-7351
Non-work interests include food, birds and athletics. And memes!
Research group of the Institute of Organic Chemistry
@univienna - https://mateoslab.com
Photochemist, former Leopoldina Postdoc, Husband, Father of 2, Chemistry Professor @ JGU Mainz, board member of the GDCh photochem. section https://www.ak-kerzig.chemie.uni-mainz.de/
A chemist believing the power of molecules. Synthetic chemistry, molecular nanocarbon science, catalysis, and chemical biology. Be unique, go crazy!
kenichiro.itami@riken.jp
https://itami-lab.com/?lang=en
Peptide chemist studying RNA-binding proteins @ Max Planck Institute of Molecular Physiology, Dortmund, Germany