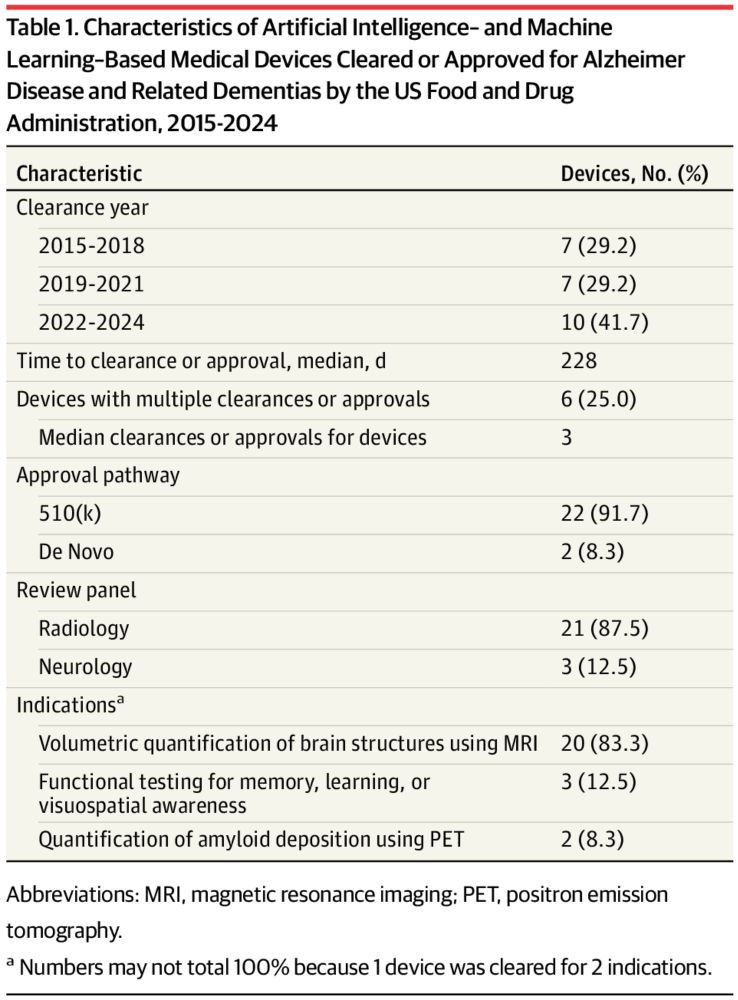
Lack of transparency raises concerns regarding underperformance, underdiagnosis, & inequalities in care recommendations. Enforcing data transparency guidelines can help promote safe use of devices. (3/4)
07.08.2025 18:00 — 👍 0 🔁 0 💬 1 📌 0
Key point: Transparency of evidence across demographics for AI/ML devices was limited. Transparency is important for understanding appropriate application, esp for demographic representativeness of training & validation datasets. (2/4)
07.08.2025 18:00 — 👍 0 🔁 0 💬 1 📌 0
ICMYI: AI/ML-enabled medical devices have potential for earlier diagnosis & symptom monitoring for Alzheimer’s disease & other dementias. A new @jama.com current study led by @hopkinsmedicine.bsky.social student Krista Chen looks at demographic representativeness of these devices (1/4)
07.08.2025 18:00 — 👍 0 🔁 0 💬 1 📌 0

Exclusion of Older Adults from Obesity Treatment Pivotal Trials of GLP-1RAs and GIP/GLP-1RAs - Journal of General Internal Medicine
Journal of General Internal Medicine -
This team comprised of @yaleschoolofmed.bsky.social Alissa Chen, @keckschoolusc.bsky.social Yixuan Liang, @yaleschoolofmed.bsky.social Kasia Lipska, and CRRIT Co-Directors @jsross119.bsky.social and @reshmagar.bsky.social
Full analysis: link.springer.com/article/10.1...
01.08.2025 14:20 — 👍 2 🔁 1 💬 0 📌 0
Many older adults have obesity, yet few were included in clinical trials for GLP1s. Study exclusion criteria (depression, prior cancer) may contribute to the under-enrollment of adults ≥ 65 years (1/2)
01.08.2025 14:20 — 👍 0 🔁 0 💬 1 📌 0

Regulatory change could improve biosimilar access in the US
Tiffany E Jiang , Reshma Ramachandran , and Joshua J Skydel argue that US requirements for interchangeability are not supported by evidence and hamper use of biosimilars
Biological medicines (biologi...
In a recently published article in @bmj.com, @yaleschoolofmed.bsky.social student Tiffany Jiang, CRRIT Co-Director @reshmagar.bsky.social & Joshua Skydel (Yale Rheumatology) investigate how eliminating these requirements can increase access to biosimilars (2/2)
🔗 www.bmj.com/content/390/...
30.07.2025 14:48 — 👍 1 🔁 1 💬 0 📌 0
Biosimilars provide low-cost options for patients and expanded access to treatment, but use of these treatments is hampered by @fda.gov requirements for interchangeability (1/2)
30.07.2025 14:48 — 👍 0 🔁 0 💬 1 📌 0

Compounded GLP-1 Receptor Agonists: Opportunity for Compassionate Conversation - Journal of General Internal Medicine
Journal of General Internal Medicine -
In a new @journalgim.bsky.social viewpoint, @yaleschoolofmed.bsky.social & Yale Internal Medicine Ashwin Chetty and Dr. Alissa Chen Chen describe the importance of clinicians proactively discussing these treatments with patients. (2/2)
Full viewpoint: link.springer.com/article/10.1...
29.07.2025 13:46 — 👍 0 🔁 0 💬 0 📌 0
Many clinicians do not recommend compounded GLP-1RAs and are uninformed when patients seek these medications, which can leave patients vulnerable to misinformation. (1/2)
29.07.2025 13:46 — 👍 0 🔁 0 💬 1 📌 0
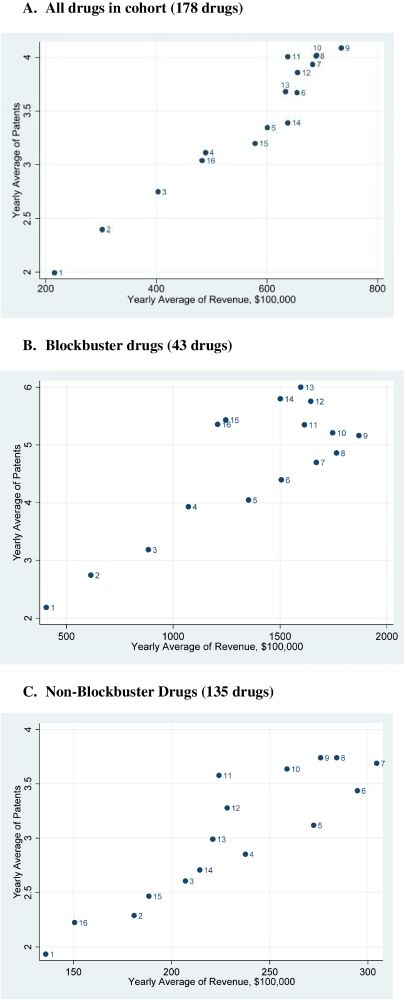
High brand-name drug prices fall once a generic enters the market. In a new @journalgim.bsky.social article, Ravi Gupta, CRRIT Co-Director @jsross119.bsky.social, and colleagues from @portalresearch.org assess associations between patents, revenue, and generic competition.
21.07.2025 15:35 — 👍 0 🔁 2 💬 1 📌 0
Dr. Ross noted these letters “would allow other sponsors & scientists to better understand what precluded drug approval — was it safety concerns or lack of efficacy — & ideally advance on that’ to bring new drugs to market” (2/2)
15.07.2025 18:00 — 👍 0 🔁 0 💬 0 📌 0

F.D.A. Posts Collection of Letters Outlining Concerns About New Drugs
ICYMI: @fda.gov released a limited set of denial letters for drugs that were ultimately approved. CRRIT Co-Director @jsross119.bsky.social recently told @nytimes.com why these letters are important (1/2)
🔗 www.nytimes.com/2025/07/10/h...
15.07.2025 18:00 — 👍 1 🔁 0 💬 1 📌 0


CRRIT Co-Director @reshmagar.bsky.social provided public comments at the Prescription Drug User Fee Act (PDUFA) VIII Kickoff Meeting @fda.gov yesterday. Dr. Ramachandran discussed how the user fee process could be reformed to be more patient-centered & transparent.
15.07.2025 14:10 — 👍 2 🔁 1 💬 0 📌 0

Validating Surrogate Endpoints to Support FDA Drug Approval
As surrogate markers are increasingly being accepted by FDA to support approval of new drugs and biologics, it is imperative that patients and clinicians understand whether such novel endpoints are re...
Surrogate markers are increasingly being used to support @fda.gov approvals instead of measuring clinical outcomes. In a @scientistsorg.bsky.social memo @reshmagar.bsky.social, Joshua Wallach & @jsross119.bsky.social outline how FDA can make transparent & strengthen evidence. fas.org/publication/...
10.07.2025 21:10 — 👍 3 🔁 1 💬 0 📌 0

F.D.A. to Use A.I. in Drug Approvals to ‘Radically Increase Efficiency’
The @fda.gov says it will “radically increase efficiency” with AI and embrace “radical transparency.” But as @reshmagar.bsky.social told @nytimes.com, agency officials recently kicked off a six-city listening tour—with Pharma CEOs—behind closed doors. Here’s the scoop:
20.06.2025 21:43 — 👍 1 🔁 1 💬 0 📌 0
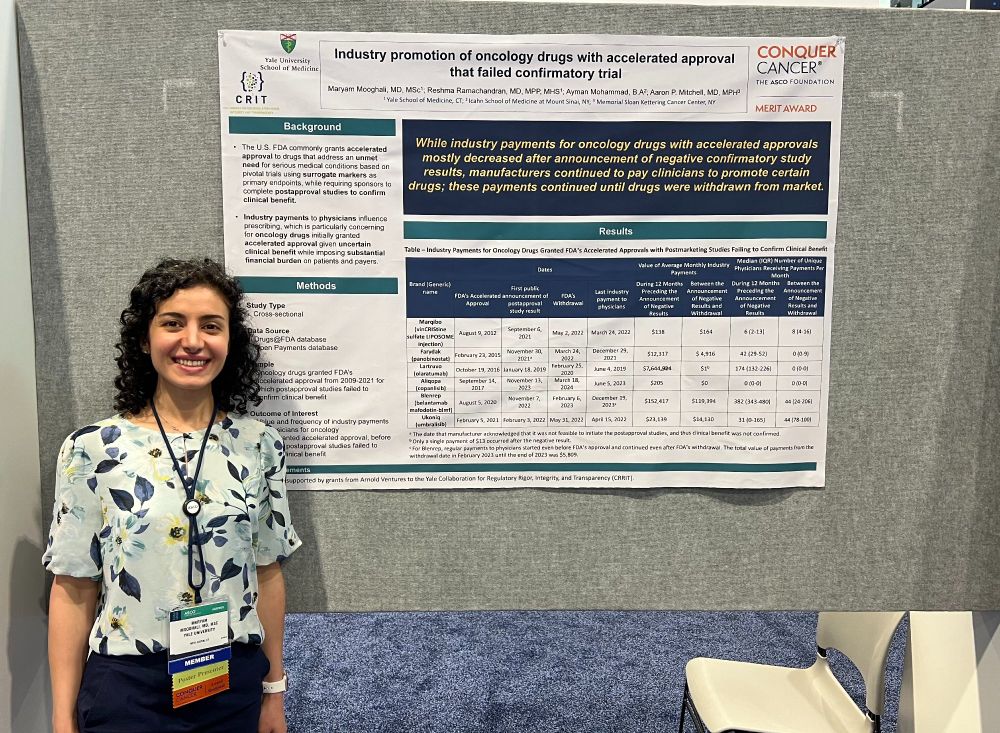
Do companies continue promoting drugs granted
@fda.gov accelerated approval even after failed confirmatory trials? Yes—though less aggressively, finds Dr. Maryam Mooghali’s #ASCO25 Merit Award-winning research.
🔗 meetings.asco.org/abstracts-pr...
02.06.2025 19:07 — 👍 3 🔁 0 💬 0 📌 0
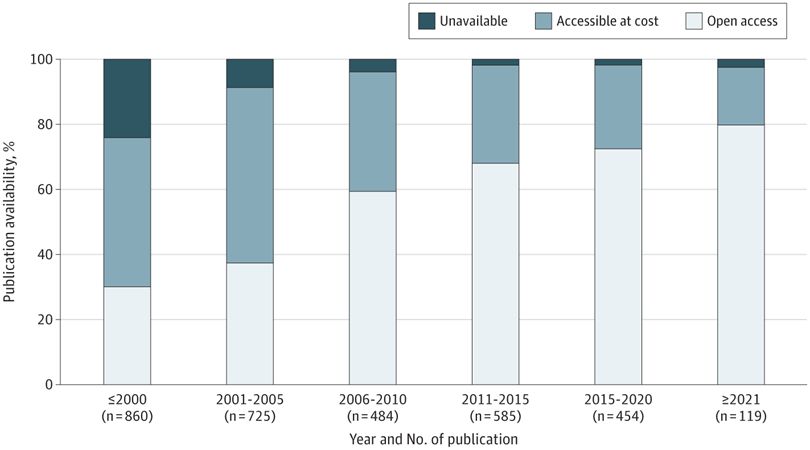
...her team found that information published in scholarly journals is becoming more accessible over time (see below ⬇️)—giving patients and their caregivers more agency to make the health decisions best for them. #InfoAsHealth
@jsross119.bsky.social @genpatient.bsky.social
22.05.2025 18:49 — 👍 0 🔁 0 💬 0 📌 0
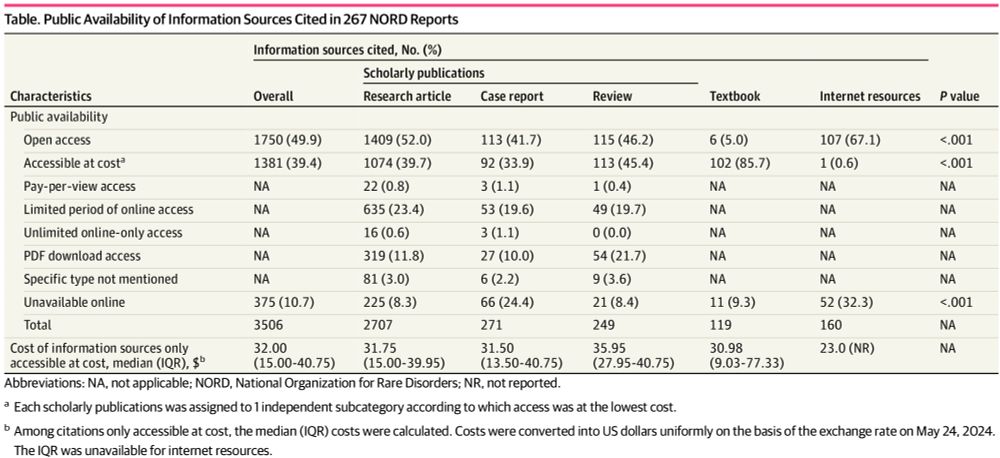
Access to Information Cited in National Organization for Rare Disorders Reports
This cross-sectional study describes the availability and access cost to the public of research findings and other clinical insights relevant to rare diseases.
For patients with rare diseases, high-quality health info can be scarce. Research led by CRRIT post-doc Mengyuan Fu, PhD, on @nordrare.bsky.social’s free online disease reports found that 50% of sources cited were unavailable online or behind paywalls (~$168 per report). But... 🧵
22.05.2025 18:47 — 👍 1 🔁 0 💬 1 📌 1

Congrats to Reshma Ramachandran MD, MPP, MHS (Yale NCSP '20–'22), winner of the 2025 Bernard Lown Award for Social Responsibility! 👏 A powerful voice for health equity. Learn More: bit.ly/reshma-lown2025
#HealthEquity #SocialResponsibility #YaleNCSP #PharmaReform #PublicHealth@reshmagar.bsky.social
14.05.2025 19:32 — 👍 5 🔁 2 💬 0 📌 0

Implications for Public Health Regulation if Chevron Deference Is Overturned
This Viewpoint describes implications for medicine and public health if the US Supreme Court decides to overturn or narrow Chevron deference.
After the Supreme Court overturned Chevron deference last year, courts—not agencies—get final say on ambiguous laws. But judges often lack scientific expertise, and the ruling on lab-developed test shows the risks.
For more on public health post-Chevron:
05.05.2025 16:41 — 👍 2 🔁 1 💬 0 📌 0

A court decision on lab-developed tests sets a dangerous precedent
An under-the-radar court ruling may be among the most significant harbingers of the crises to come for the FDA and public health.
In March, a Texas judge tied @fda.gov’s hands on its ability to regulate lab-developed tests—to prevent, for example, another Theranos. In @statnews.com, CRRIT’s @ktkadakia.bsky.social, @jsross119.bsky.social, & @reshmagar.bsky.social explain the dangers of this ruling:
05.05.2025 16:40 — 👍 3 🔁 3 💬 1 📌 0
Our co-Director's research & policy work was featured by @yaleschoolofmed.bsky.social including what led to her & @jsross119.bsky.social re-establishing CRRIT which brings together expertise across campus to study how drugs, devices, etc are regulated & how that can be improved for patients. #MedSky
02.05.2025 19:44 — 👍 2 🔁 1 💬 0 📌 0
This study was authored by Dr. Maryam Mooghali, Dr. Sanket Dhruva, Hollin Hakimian, Dr. Vinay Rathi, Kushal Kadakia (@ktkadakia.bsky.social), and Dr. Joseph Ross (@jsross119.bsky.social).
02.05.2025 18:33 — 👍 0 🔁 0 💬 0 📌 0
Nonconcurrent controls can be justified—but they risk bias, selective reporting, and confounding. To protect patients and earn trust in nonconcurrent controls, @fda.gov could require justifications of and disclose why these controls are used.
02.05.2025 18:32 — 👍 0 🔁 0 💬 1 📌 0
We found that:
- Among 88 high-risk devices approved,
- 52 (59.1%) used ≥1 trial with nonconcurrent controls, and
- The @fda.gov docs of just 3 (3.8%) included justifications for using nonconcurrent controls.
02.05.2025 18:31 — 👍 0 🔁 0 💬 1 📌 0
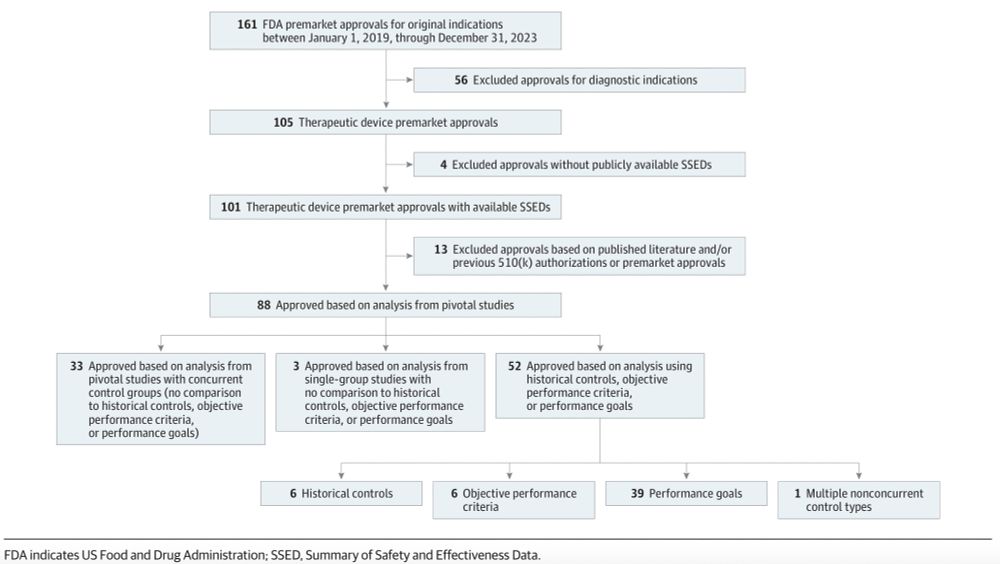
In this study, we reviewed @fda.gov approvals of high-risk therapeutic devices from 2019–2023 to see how often nonconcurrent controls were used—and whether @fda.gov justified their use.
02.05.2025 18:30 — 👍 0 🔁 0 💬 1 📌 0
The National Clinician Scholars Program @Yale. Preparing clinician leaders who will generate & use scientific evidence to improve health & healthcare for all.
Opinion editor at STAT. Send your conversation-starting medicine/life sciences opinion pieces my way! https://www.statnews.com/first-opinion-submission
Co-founder & Executive Editor, STAT (www.statnews.com)
@statnews.bsky.social
Longtime reporter/editor NYT
Covering science, medicine & business for STAT
Reach out on Signal: JasonMast.05
I write about substance use and the drug overdose crisis for @statnews.com
https://www.statnews.com/staff/lev-facher/
Reporter at CNN writing about health policy, politics and Washington D.C.
Signal: sarah_owermohle.07
Reporter @statnews.com covering drugs and the biopharma industry | signal: elaineywchen.70
I cover health equity for STAT. Yes my job is harder these days. Signal ID: usha.22
Affordable, quality health care. For everyone.
About Us: https://buff.ly/3PTPkDt
Health econ & Rx drug pricing. 💵💲💉💊Opinions my own.
Science and global health reporter for The New York Times
https://www.nytimes.com/by/apoorva-mandavilli
Signal: amnyt.91
MEDICINE / INFECTIOUS DISEASES / EPIDEMIOLOGY / JOURNALISM
🔹 Senior Fellow & Editor-at-Large for Public Health at @KFFhealthnews.bsky.social
🔹 Medical Contributor for CBS News
🔹 Prof/Doc at NYU Grossman School of Medicine & Bellevue Hospital
ER doctor at Brigham and Women's in Boston, Harvard Medical School.
Words in: NY Times, WaPo, Atlantic, Slate, CNN. I'm also "editor-in-chief" of MedPage Today and write "Inside Medicine" on Substack:
https://insidemedicine.substack.com/subscribe
Director, CIDRAP
New episodes of our podcast on the latest infectious disease threats: https://www.cidrap.umn.edu/osterholm-update
Emergency physician & Dean of the Yale School of Public Health. Gun violence prevention researcher. Incorrigible optimist (because we can and do create change, together). Mom of two teens. GO BILLS. @meganranney at the other place 🛟🩺📉📈🧪
Editor in Chief, Blood Cancer Journal; Professor, Mayo Clinic; Associate Editor, Mayo Clinic Proceedings; Oncologist, Cancer Research, Myeloma.
Health care business reporter for @statnews.com and its Health Care Inc. newsletter. Contact: bob.herman@statnews.com or bobjherman.09 on Signal. Sign up: https://www.statnews.com/signup/health-care-inc/
Co-Chair, Arnold Ventures
Author (The Fault in Our Stars, The Anthropocene Reviewed, etc.)
YouTuber (vlogbrothers, Crash Course, etc.)
Football Fan (co-owner of AFC Wimbledon, longtime Liverpool fan)
Opposed to Tuberculosis