
Great to see this out! @fabiandankert.bsky.social and I summarise some of our favourite papers in main group chemistry over the last year
gdch.app/article/haup...
@maltefischer.bsky.social
"Fishing" around in early transition metal and main group chemistry // TT Ass. Prof. @ Georg-August-Universität Göttingen // Liebig fellow // https://www.uni-goettingen.de/de/674212.html

Great to see this out! @fabiandankert.bsky.social and I summarise some of our favourite papers in main group chemistry over the last year
gdch.app/article/haup...

📣Our new paper is out in @jacs.acspublications.org (our very first one)!🥳
We show how a redox-active ligand unlocks C–X bond scission at an ambiphilic T-shaped Bi(III) compound. A big collaborative effort with my brilliant colleagues from Berlin.
👉 pubs.acs.org/doi/10.1021/...
The group will soon be advertising a 3-year PDRA position @imperialchemistry.bsky.social. The successful candidate will research the synthesis and reactivity of complexes with alkaline earth-metal bonds. Start date: 03/26. Please get in touch for infomal inquries.
08.01.2026 15:26 — 👍 18 🔁 18 💬 0 📌 0Just 4 weeks left to apply for the PhD position in our research group! Deadline: 31 January. Don’t miss out, apply today :)
05.01.2026 10:05 — 👍 5 🔁 9 💬 0 📌 0What's my #CSDPacked @ccdc.cam.ac.uk? My publishing name is Malte Fischer.
10.12.2025 15:35 — 👍 1 🔁 0 💬 1 📌 0Huge congratulations!🥳
09.12.2025 15:05 — 👍 1 🔁 0 💬 1 📌 0
TIRE CHANGE: First Mg(I) complex with a Cp* ligand enables facile ligand exchange. Reaction with RONa gives (BDI)Mg-MgOR and Cp*Na. This paves the way for syntheses of many new asymmetric Mg(I) complexes. @angew_chem onlinelibrary.wiley.com/doi/epdf/10....
17.10.2025 13:12 — 👍 34 🔁 9 💬 0 📌 1
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.202515545#
Our group's very first ACIE paper is out!🥳
Awesome work by Soti and a great cooperation with the Bittl group and @franemmerling.bsky.social.
Dedicated to @christianlimberg.bsky.social and Franc Meyer
@humboldtuni.bsky.social @manchester.ac.uk
onlinelibrary.wiley.com/doi/full/10....
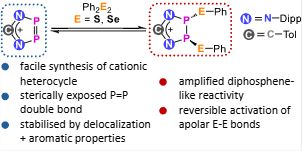
First post, first publication!
I’m over the moon to share the first publication from our small research group! 🚀 pubs.rsc.org/en/content/a... @chemcomm.rsc.org
Jan explored a funky P=P-containing heterocycle and its reversible! bond activation chemistry, with DFT insights from Francesco. #newPI

Thrilled to share a first publication of the group! We report on cadmium aluminyls that find application as Cd-nucleophiles and(!) Al(I)-Transfer reagents. Now out in @jacs.acspublications.org ! 🥳👍
Thanks a lot to @chemieverband.bsky.social for the generous funding. pubs.acs.org/doi/10.1021/...


Honored to welcome guests from the prestigious Tsinghua University in Beijing tsinghuauniversity.bsky.social for a fruitful exchange with representatives of the Faculty of Chemistry and the CRC 1633 in Göttingen. Many thanks for the inspiring discussions and the successful collaboration!
31.07.2025 17:28 — 👍 8 🔁 1 💬 0 📌 0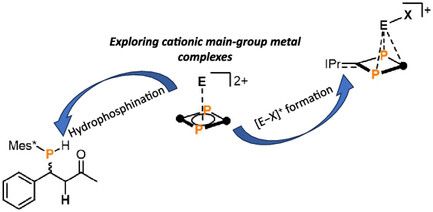
Our latest publication reports the successful isolation of π-stabilized tetryliumylidenes [E–X]+ starting from pyramidanes based on a biradicaloid ligand . This milestone was made possible thanks to David’s relentless efforts . Proud of the team!
26.07.2025 13:35 — 👍 15 🔁 2 💬 1 📌 0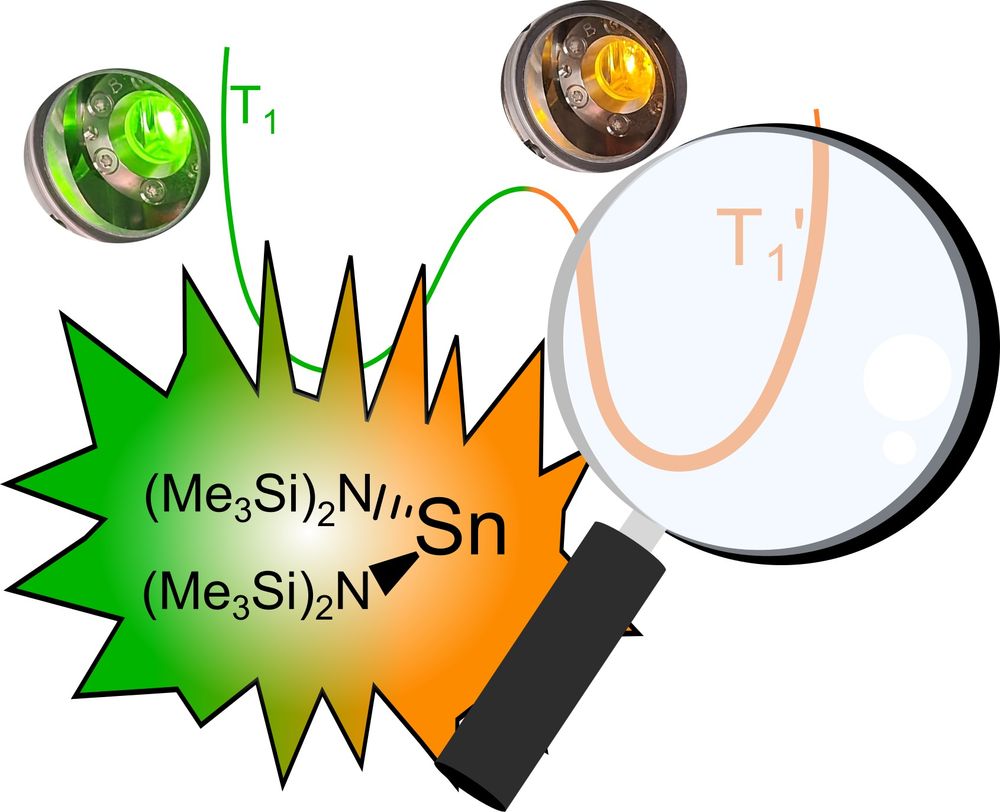
Did you know that #stannylenes can shine orange and green?
Philipp elucidated the mechanism of the dual #emission, the #excited state #dynamics, #excimer formation and light-induced bond #homolysis. #Maingroup #tin #photophysics #photochemistry onlinelibrary.wiley.com/doi/10.1002/anie.202510044
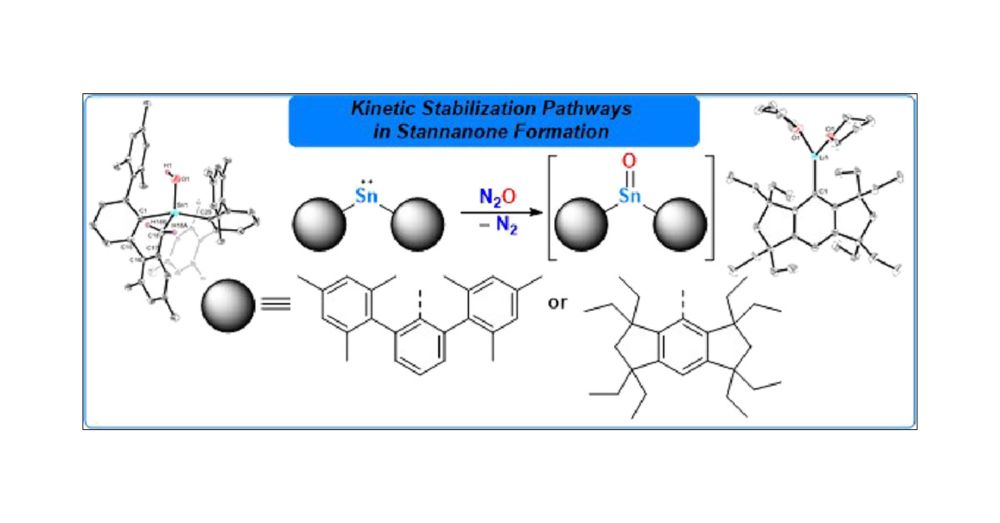
Excited to share our contribution to the special issue "Organometallic Chemistry Beyond the Transition Metals: Fundamentals and Applications of the P-Block" – now published @Organometallics! Check it out 👉 @pubs.acs.org #ChemSky
pubs.acs.org/doi/10.1021/...

Check out Fabio's next paper on the Nature of the Heavy Formal Double Bonds As=Ch, Sb=Ch and Bi=Ch (Ch = S, Se, Te) in NCN-Pincer Supported Arsinidene, Stibinidene and Bismuthinidene Chalcogenides - now published in Chemical Science pubs.rsc.org/en/content/articlelanding/2025/sc/d5sc03320a
18.07.2025 06:56 — 👍 16 🔁 4 💬 0 📌 0
Thrilled of having contributed to this investigation of low-valent aluminium species and their unique reactivity with azides. Great collaboration with the Stalke and Krawczuk groups! onlinelibrary.wiley.com/doi/10.1002/...
08.07.2025 15:41 — 👍 12 🔁 2 💬 0 📌 0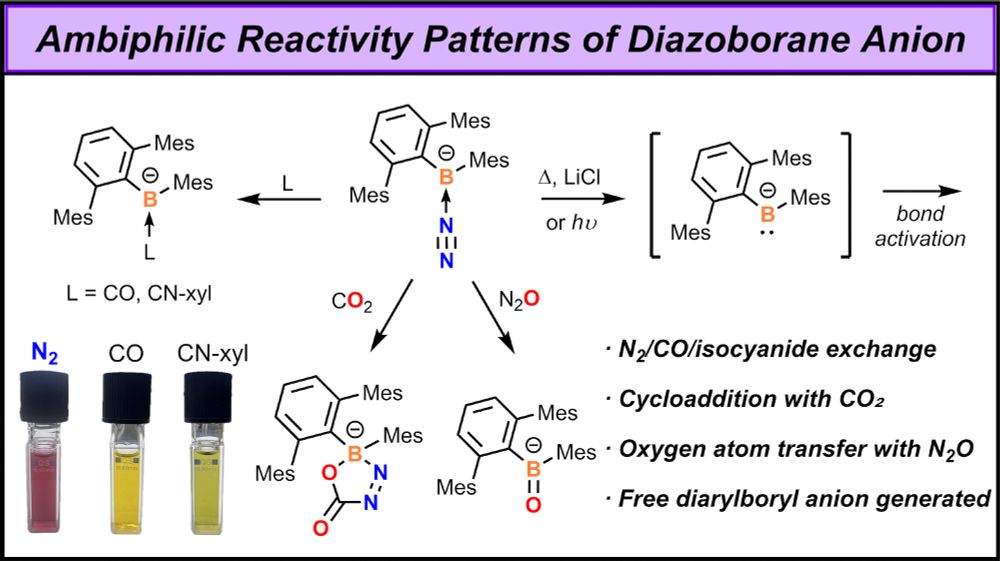
Our manuscript on unlocking the ambiphilicity of boryl anions is now published in JACS @jacs.acspublications.org (pubs.acs.org/doi/full/10....)! Chonghe, Junyi, Xibao, and coworkers report the synthesis and reactivity of the first anionic diazoborane! #maingroup #boron @gilliardgroup.bsky.social
10.06.2025 19:13 — 👍 34 🔁 5 💬 0 📌 1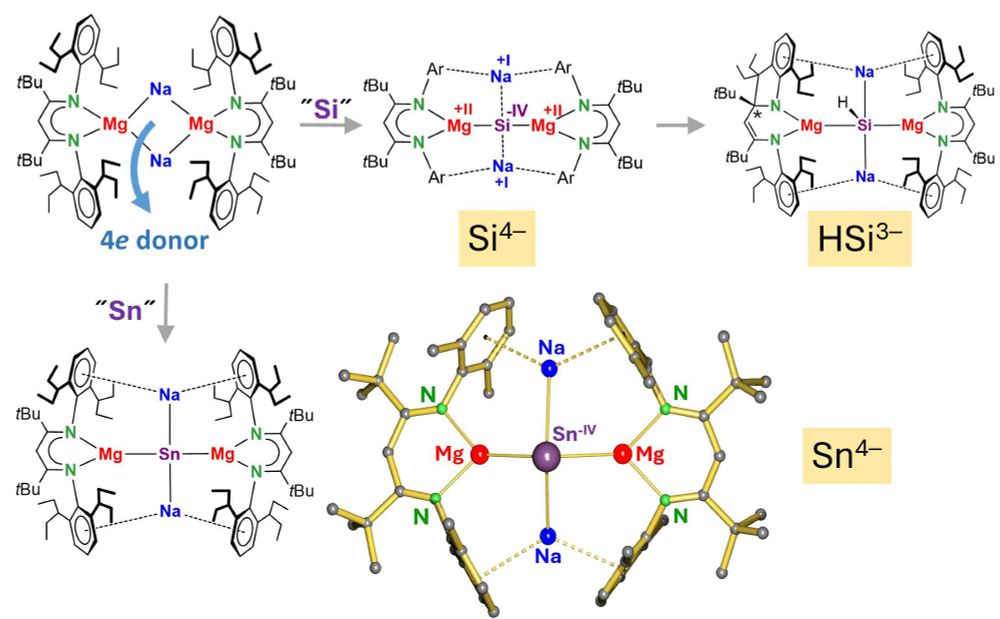
LOW-VALENT Si, Ge, Sn, Pb: HOW LOW CAN WE GO???
Hydrocarbon-soluble Mg(0) reduces Si all the way to Si(4-). Unfortunately not stable and decomposing to the HSi(3-) anion. However, Sn(4-) is stable and functions as 4-fold Nu or 8e reducing agent. Preprint: shorturl.at/4mH0O
🔓Explore recent #OpenAccess work by @maltefischer.bsky.social, @townrowresearch.bsky.social and colleagues on the isolation of the parent triplet titanocene via NHC stabilisation🔥
pubs.rsc.org/en/content/a...
📍@unigoettingen.bsky.social and @kit.edu
🧪
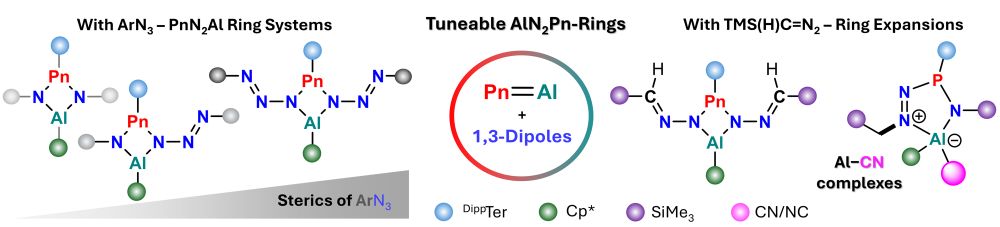
Rreactivity of Pnictaalumenes towards 1,3-Dipole Molecules fresh out in @angewandtechemie.bsky.social Tim, Edgar and Leonie uncover unique insertion chemsitry giving a plethora of Al,P,N-heterocycles! Again a fruitful collaboration with the Braunschweig Group!
doi.org/10.1002/anie...

Dalton Transactions promotional graphic for the HOT articles collection.
This year's HOT articles collection is now online🔥
This collection represents the top 10% of research published in our journal each quarter. Congratulations to all the authors whose articles are featured in this collection👏
pubs.rsc.org/en/journals/...
#Chemsky 🧪
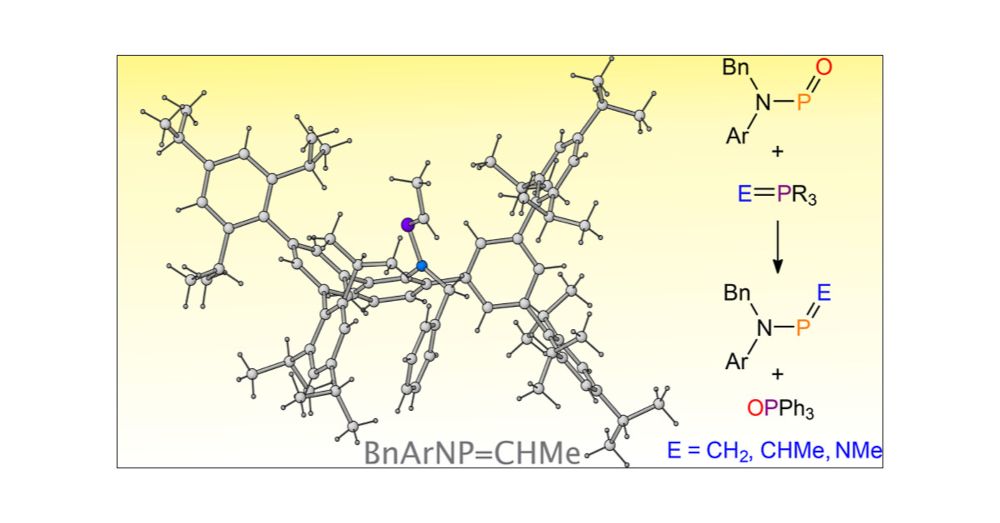
Fresh out of the oven. Check out Chenyang’s most recent work in JACS (@jacs.acspublications.org). We explore the reactivity of a phosphinidene oxide as the electrophilic partner in Wittig transformations. Hope you enjoy.
pubs.acs.org/doi/full/10....
Such beautiful chemistry, Ian!😊
21.03.2025 07:03 — 👍 2 🔁 0 💬 0 📌 0Many thanks, Sakya!☺️
21.03.2025 06:56 — 👍 1 🔁 0 💬 0 📌 0Huge congratulations to Jennifer and Navutheya, whose master’s and bachelor’s theses are summarized here, and to Christopher for his outstanding help with SCXRD! 2/2
19.03.2025 17:48 — 👍 3 🔁 0 💬 0 📌 0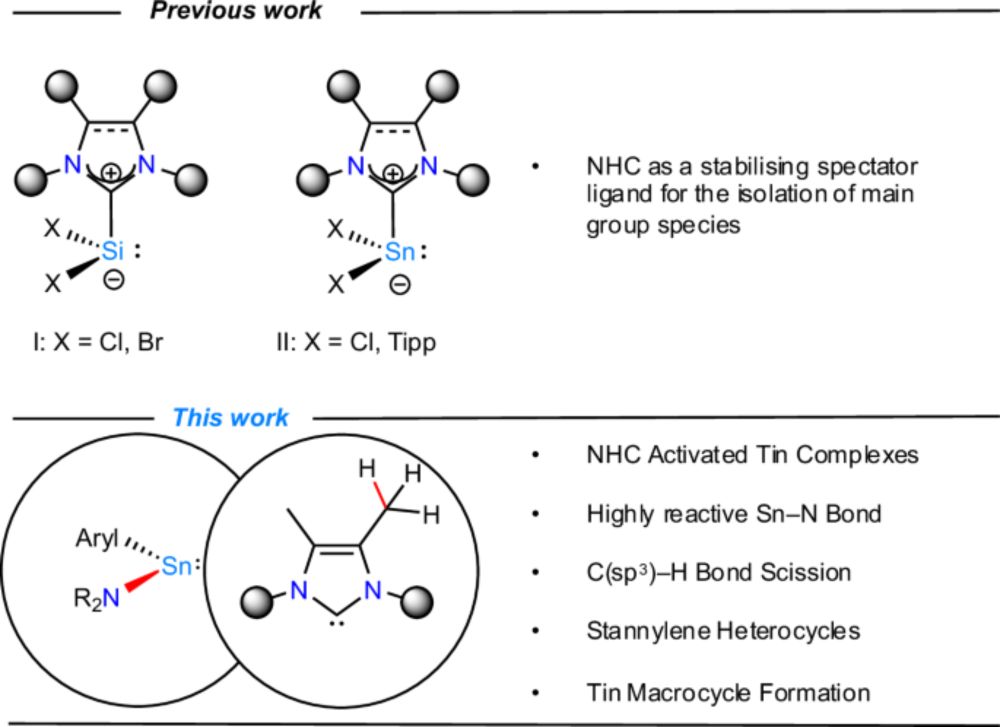
Thrilled to share our latest work from the lab, in collaboration with @townrowresearch.bsky.social , exploring the fusion of heteroleptic amido-substituted stannylenes and NHCs! #chemsky www.nature.com/articles/s41... 1/2
19.03.2025 17:48 — 👍 24 🔁 5 💬 2 📌 1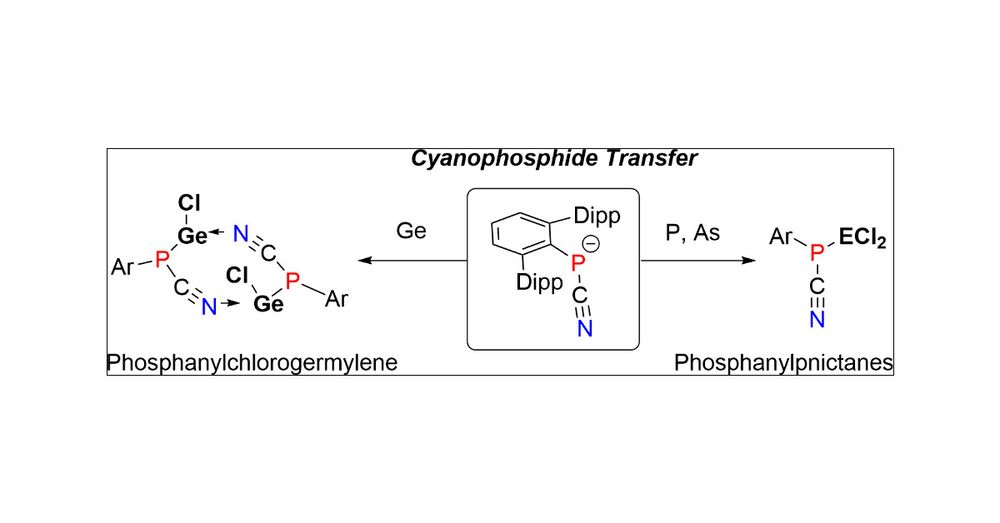
Ayu is keeping it up! This piece is about ArPCN- transfer to give Germylenes and phosphapnictanes! Thanks @maltefischer.bsky.social and @fabiandankert.bsky.social for their help! Thanks Organomteallics for the smooth proceedings! #31p #phosphorus
pubs.acs.org/doi/10.1021/...
Some new fantastic chemistry from Christian and team!😊
13.03.2025 19:19 — 👍 3 🔁 0 💬 0 📌 0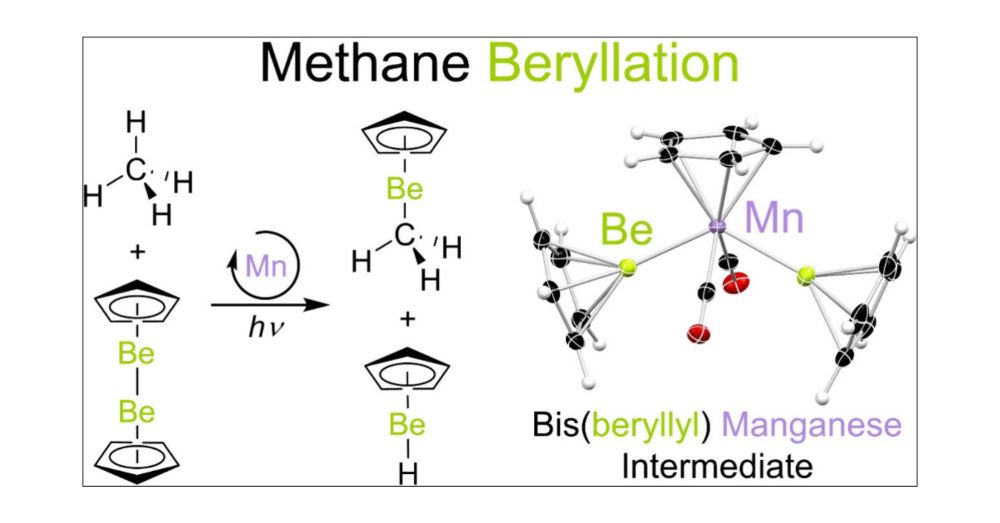
Catalytic methane functionalization and all it takes is... beryllium! We also compare the mechanisms of methane beryllation and borylation to reveal why Be finds it so easy @pubs.acs.org pubs.acs.org/doi/10.1021/...
11.03.2025 19:45 — 👍 66 🔁 11 💬 2 📌 1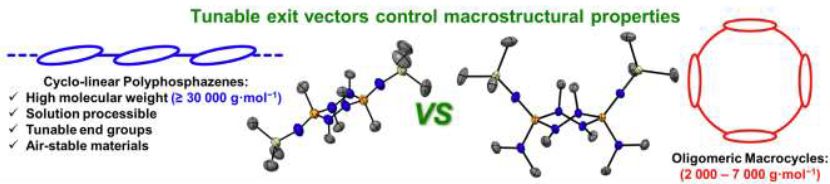
🚨Two projects out from our lab today (links fixed!)
First up @angewandtechemie.bsky.social
Macro/molecular chemistry of cis/trans P2N4 rings.
Congrats @michaelland.bsky.social w/ @jasonmasuda.bsky.social for Xray
onlinelibrary.wiley.com/doi/10.1002/...
cc @dalchemistry.bsky.social