Substrate-Photocatalyst Reactivity Matching Enables Broad Aryl Halide Scope in Light-Driven, Reductive Cross-Electrophile Coupling Using 13C NMR as a Predictor
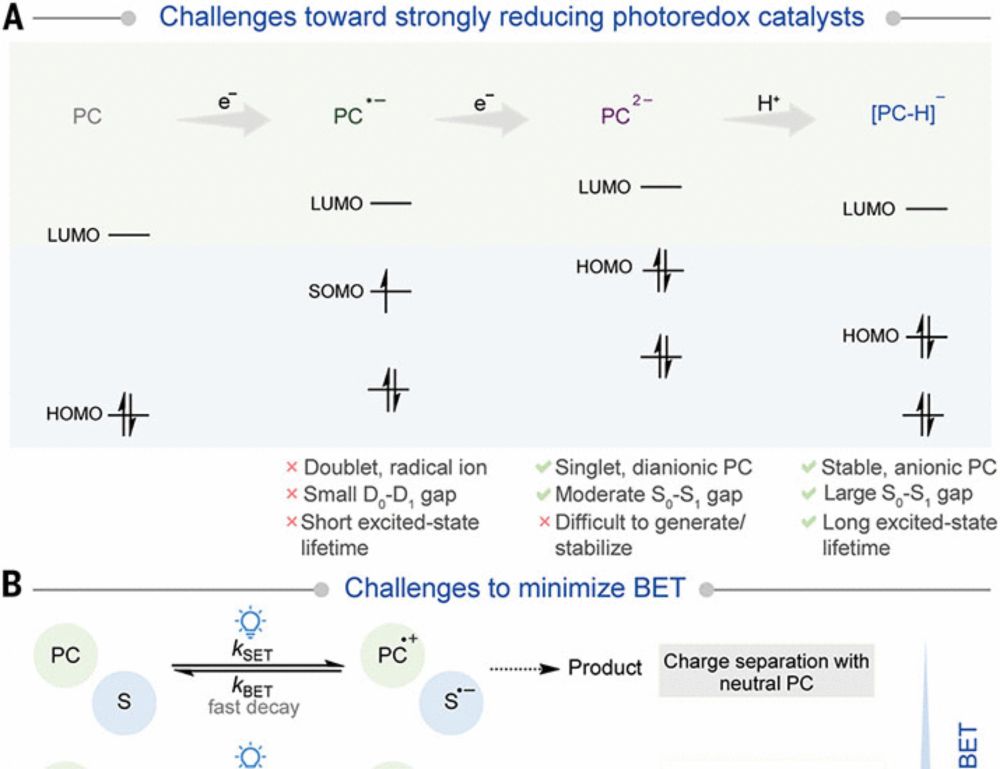
The cross-electrophile coupling between aryl and alkyl halides is an important synthetic tool in the creation of Csp2–Csp3 bonds from abundant building blocks. These couplings have traditionally relied on thermally driven, reductive couplings using metallic Zn/Mn to generate reactive low-valent nickel species. Recent work has expanded this reactivity to visible light-driven methodologies utilizing a range of photocatalysts and terminal reductants. However, early photocatalyzed approaches required the use of precious metal photocatalysts and stoichiometric amounts of a costly silane. The work described herein expands photoredox catalyzed cross-electrophile coupling with a focus on the use of organic photocatalysts and an inexpensive, readily available sacrificial electron source (triethanolamine). To overcome limitations in substrate scope arising from redox incompatibilities between photocatalyst and substrate, we introduce two sets of conditions that minimize unwanted substrate-specific side reactivity. These synthetic protocols enable cross-electrophile couplings of challenging aryl bromide substrates, such as unprotected bromoindoles and 2-bromopyrimidines. We found that the propensity of aryl halide reagents to undergo side reactions is correlated with electronic parameters: the C–Br 13C chemical shift of aryl bromides is a robust predictor for this reactivity and enables facile reaction condition selection.
Congrats to Cam, Zach, Graham, Raul, Amreen, Claire, Trevor, Max, and David on their publication in ACS Catalysis!
pubs.acs.org/doi/full/10....
02.05.2025 19:38 — 👍 2 🔁 0 💬 0 📌 0
Congrats to SuPRCats Prof. Zach Wickens (@zachwickens.bsky.social) for being selected as a Dreyfus Teacher-Scholar for 2025 and Prof. Garret Miyake (not-on-bluesky) for being selected for CSU's scholarship impact award! Check out the details below!
02.05.2025 17:32 — 👍 1 🔁 1 💬 1 📌 0
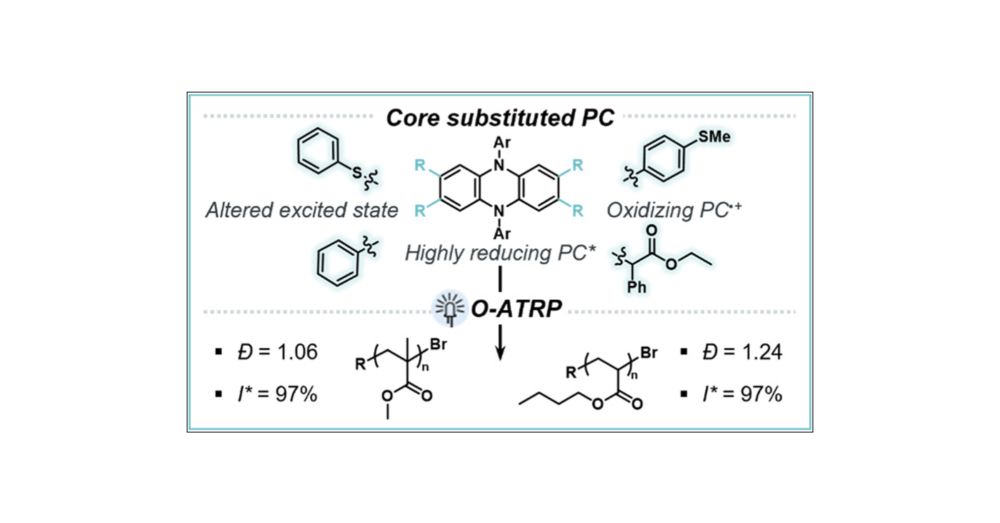
Influence of Dihydrophenazine Photoredox Catalyst Excited State Character and Reduction Potentials on Control in Organocatalyzed Atom Transfer Radical Polymerization
The development of N,N-diaryl dihydrophenazine organic photoredox catalysts (PCs) has enabled numerous examples of organocatalyzed atom transfer radical polymerization (O-ATRP) of methyl methacrylate (MMA) monomer to polymers with low dispersity (Đ < 1.30) and near-unity initiator efficiency (I* ∼ 100%), as well as small molecule synthesis. In this work, we investigate the influence of core substitution (CS) by alkyl, aryl, and heteroatom groups on singlet excited state reduction potential (ES1°*). We observe that a highly reducing ES1°* is in part a result of a locally excited (LE)-dominated hybridized local and charge transfer (HLCT) excited state in CS PCs, which is influenced by the identity of the core substituent. Additionally, the PCs that possess a LE-dominated HLCT character maintain a relatively oxidizing PC radical cation oxidation potential (E1/2) for deactivation in O-ATRP compared to fully LE PCs reported in prior work. For example, a thiophenol core substituted (heteroatom CS, HetCS) PC shows the most negative ES1°* (−2.07 V vs SCE), more LE character (Stokes shift = 124 nm), and has an oxidizing PC radical cation (E1/2 = 0.30 V vs SCE). The CS PCs with improved properties, including more negative ES1°*, perform best in O-ATRP of MMA with the HetCS PC showing the best control in both DMAc (Đ = 1.08, I* = 89%) and EtOAc (Đ = 1.06, I* = 97%). Additionally, the HetCS PC was found to mediate the controlled polymerization of n-butyl acrylate (n-BA) (Đ = 1.24, I* = 97%), which has remained challenging in O-ATRP without supplemental deactivation strategies. An aryl CS PC was found to have moderate control as low as 1 ppm PC, indicating facilitation of low PC loadings (Đ = 1.33, I* = 69%). The relationship between excited state character, ES1°*, and polymerization control observed in this work provides a foundation for increasing the utility of phenazine PCs across photoredox catalysis.
Congrats to former SuPRCat-er and recent grad Katrina on her publication in ACS Catalysis! pubs.acs.org/doi/10.1021/...
11.03.2025 21:59 — 👍 3 🔁 2 💬 0 📌 0
If anyone knows of other public forums where we could share this work, please DM us!!
29.01.2025 21:18 — 👍 1 🔁 0 💬 0 📌 0

Science on Tap yesterday was a hit! Congrats to Ally and Jess for sharing their work with our community!
29.01.2025 21:17 — 👍 1 🔁 1 💬 1 📌 0
Check out this Chemistry World piece highlighting exciting work from our researchers on using visible-light-powered catalysts to clean up PFAS!
03.12.2024 17:18 — 👍 6 🔁 2 💬 0 📌 0


Happy Fluorescence Friday! Check out these beautiful Pt(II) complexes phosphorescing under UV light!
22.11.2024 17:12 — 👍 17 🔁 2 💬 0 📌 0
Second paper described is from a few of our own researchers!!
21.11.2024 23:07 — 👍 4 🔁 1 💬 0 📌 0
YouTube video by Colorado State University
Sustainable Photoredox Catalysis l CSU Chemistry Innovation Shines Bright
💡 SuPrCat 💡
www.youtube.com/watch?v=MprZ...
21.11.2024 03:57 — 👍 2 🔁 0 💬 0 📌 0
NIH K99/R00 MOSAIC Scholar, Reisman Lab Caltech | 2022 PhD Brown Lab IU | BS/BA Hope College | Organic chem 🧪 aspiring academic 👩🔬 avid reader 📚 she/her
Old process chemist - I make drugs by the metric fuckton
Ready to make the free pharmaceuticals that save lives in the glorious corporation free future
Follow all scientists
Not a Dem or a Repub, but a secret 3rd thing
Ask me about drugs or chemistry
We are a non-profit communications & advocacy organization that collaborates with an expansive global alliance of organizations, businesses, & individuals to create a more just, equitable, regenerative world free of plastic pollution & its toxic impacts.
We eat, drink and breathe toxic plastics. This has to stop.
We want to turn off the plastic tap. Tackle plastic pollution at the source. We are building awareness and calls to action. We encourage new legislation and encourage investments on new materials
Ecotoxicologist, fish biologist, plastic researcher, teacher, communicator, wife, mother, lover of animals.
Steering member of The Scientists' Coalition for an Effective Plastics Treaty https://ikhapp.org/scientistscoalition/
Father/Husband | Former Assoc Prof | #PlasticPollution #PFAS #EmergingContaminants |Alum longwoodu @CofC @LSU_WABL | #DIY Enthusiast | Views Own
Currently writing An Intimate History of Plastics (Scribner)………🐌
Brown Institute for Environment and Society
Words in Orion | The Atlantic | Science 📚
Chatty re: env history of plastic, #plasticstreaty & #creativenonfiction
https://rebecca-altman.com
Chemistry: Covered. The latest news, research, features and opinion from across the chemical sciences. Published by the Royal Society of Chemistry.
https://www.chemistryworld.com/
organometallic and polymer chemistry, memes and cat pictures
Associate Professor in the Andlinger Center for Energy and the Environment @ Princeton University ⚡️🔋🌿🔌
Senior editor at ACS Energy Letters
https://www.hatzelllab.princeton.edu
A chemistry journal published by SpringerNature
.
Science News #Technology #News #Chemistry #Physics #Medicine #Astronomy #Informatics #Materials #Geoscience #LifeScience
McGill Green and Sustainable Chemistry Prof, Exec Editor @ACSSustain,President College @RSC-SRC 🇨🇦🇫🇷🇬🇧Wife, Mother, Cat lover, Gardener, Aspiring Beekeeper 🐈 🪴 🐝
IZTECH-Chemistry, I like to be Polymath(learning different subjects), Polygot(interested in the learning different languages) comments and opinions are my own RT≠ is not endorsement (he/him)
Computational Organic Chemist
@University of Manchester #CompChem 💻 ⚛️ #Catalysis
🇪🇸 → 🇬🇧 | Mum of 3 boys 🤩
Cyclist 🚴♀️ | Yogi 🧘♀️ | Amateur cook
Rock music fan 🎸
🔗 https://trujilloresearchgroup.com/
PhD student Technische Universität Berlin #ComputationalChemistry #VibrationalSpectroscopy 🇦🇷 in 🇩🇪
MSc Chemistry student @ UL 🧪🇱🇻| Laboratory assistant @ LIOS ⌬ | iChO&IMChO 2020 🥉
CONICET Assist. Researcher 🇦🇷 || Postdoc @iciqchem.bsky.social - Llobet Group | UNC-Chapel Hill - Meyer Group || surviving the libertarian wave | pro-DEI || electron transfer - spectroscopy - coordination chemistry - interfaces - electrocatalysis ||🚴🏼
Chemical Biology at TU Dresden. Bringing chromocontrol, redox cascades, and photoswitch-based imaging into biology.
https://tu-dresden.de/chemie/chembio | PI posts.
orcid.org/0000-0003-3981-651X