


 29.08.2025 17:18 — 👍 0 🔁 0 💬 0 📌 0
29.08.2025 17:18 — 👍 0 🔁 0 💬 0 📌 0
@kozlowskigroup.bsky.social
Organic chemistry research group at UPenn, interested in reactions and molecules from all perspectives, on the look out for interesting chemistry challenges.



 29.08.2025 17:18 — 👍 0 🔁 0 💬 0 📌 0
29.08.2025 17:18 — 👍 0 🔁 0 💬 0 📌 0

Another summer BBQ in the books! 2025 Kozlowski Lab bocce tournament champions to be announced. @pennchemistry.bsky.social
29.08.2025 17:17 — 👍 2 🔁 1 💬 1 📌 0
Kalyana (G4) attending the 2025 Organic Reactions and Processes Gordon Research Conference at Bryant University!
07.08.2025 20:52 — 👍 1 🔁 0 💬 0 📌 0
Pedro (G4) attending the 2025 Plastics Recycling and Upcycling
Gordon Research Conference at @snhuniversity.bsky.social!

It's a privilege to have Marisa Kozlowski from
@pennchemistry.bsky.social with us today at NUS Chemistry in Singapore. Photo credit: Yixin Lu. #organicchemistry #OrgLett

Congrats to Guillermo on being selected as August's @pennchemistry.bsky.social Person of the Month!
05.08.2025 16:11 — 👍 1 🔁 0 💬 0 📌 0



Welcome to our summer visiting students Maya, Alex, and Ella!
01.07.2025 18:07 — 👍 1 🔁 0 💬 0 📌 0
Congrats to Pedro and collaborators on their work out in ACS Applied Polymer Materials!
pubs.acs.org/doi/10.1021/...




2025 lab trip to Puerto Vallarta, Mexico! @pennchemistry.bsky.social
24.06.2025 20:56 — 👍 2 🔁 1 💬 0 📌 0We are thrilled that the next Associate Dean of Natural Sciences in the School of Arts & Sciences is our own Prof. Marisa Kozlowski! We look forward to working with Marisa, and our new Dean Mark Trodden, to advance the mission of the School together. @kozlowskigroup.bsky.social @sas.upenn.edu
16.06.2025 16:51 — 👍 3 🔁 1 💬 0 📌 0
Congrats to Dr. Cami Berlin, Emily and Chris on their work out now in JOC! @pennchemistry.bsky.social
doi.org/10.1021/acs....

Congrats to all graduates at this weekend’s
@upenn.edu graduation! Special shout-out to former group member Cami, pictured with Max from the Schelter lab

Congratulations to Dr. Cameron Berlin on successfully defending her thesis! @pennchemistry.bsky.social
09.05.2025 21:47 — 👍 1 🔁 0 💬 0 📌 0
Join us today for Cami Berlin’s PhD thesis defense at 1 PM in Carolyn Hoff Lynch Lecture Hall! @pennchemistry.bsky.social
09.05.2025 12:37 — 👍 0 🔁 0 💬 0 📌 0
Welcome back Emily (visiting high school student), to the group! 👈🏼 @pennchemistry.bsky.social
08.05.2025 19:48 — 👍 1 🔁 0 💬 0 📌 0


 30.04.2025 19:00 — 👍 1 🔁 0 💬 0 📌 0
30.04.2025 19:00 — 👍 1 🔁 0 💬 0 📌 0

Congratulations Vincent and Cindy on passing your PhD candidacy exams! @pennchemistry.bsky.social
30.04.2025 19:00 — 👍 4 🔁 0 💬 1 📌 0
Congrats to Emmanuel, Chris, and collaborators on their work out now in @JPhysChem A!
pubs.acs.org/doi/10.1021/...

The group came together and brought their inside jokes to the whiteboard. Welcome to the first draft of MCK world! @pennchemistry.bsky.social
18.03.2025 17:00 — 👍 1 🔁 1 💬 0 📌 0
Congratulations to former PhD student from the group, Dr. Houng Kang, on been promoted to Associate Professor at Chungbuk National University! @pennchemistry.bsky.social
14.03.2025 13:31 — 👍 3 🔁 1 💬 0 📌 0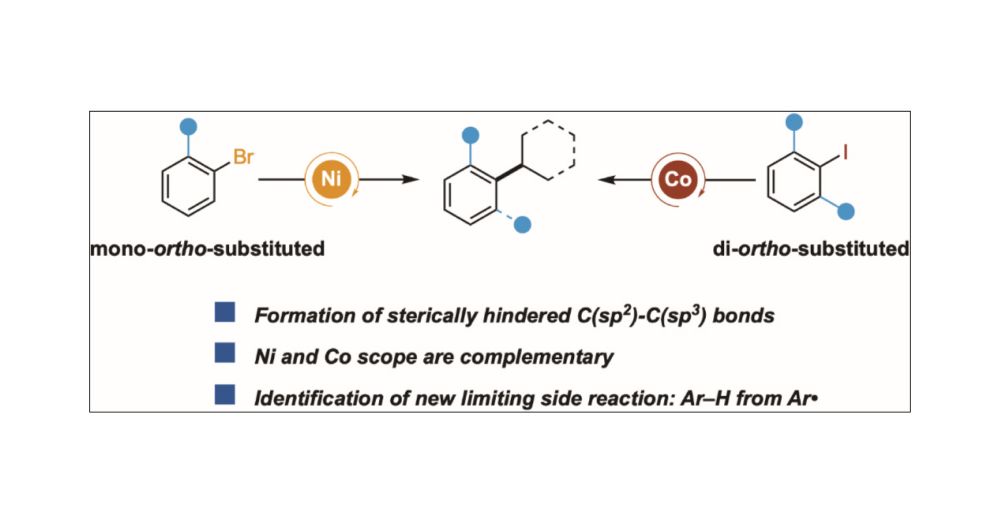
Our work on Ni- and Co-catalyzed Cross-Electrophile Coupling to Form Sterically Hindered C(sp2)–C(sp3) Bonds is now online at J.A.C.S.! @pubs.acs.org Congrats to Tianrui, Anthony, Kasturi, and our collaborators Madeline (@kozlowskigroup.bsky.social) and @novartis.bsky.social doi.org/10.1021/jacs...
07.03.2025 20:09 — 👍 12 🔁 3 💬 0 📌 1Congrats Madeline and collaborators on their work out now in J.A.C.S.!
07.03.2025 20:37 — 👍 0 🔁 0 💬 0 📌 0Hope you enjoyed your weekend @kozlowskigroup.bsky.social. It is good to see Yoshito-kun (previously @kenitami.bsky.social lab member)! I hope our paths cross again sometime.
05.03.2025 13:59 — 👍 2 🔁 1 💬 0 📌 0


Moving into our new lab spaces on the 4th floor of the Vagelos Laboratories! @pennchemistry.bsky.social
05.03.2025 13:16 — 👍 3 🔁 1 💬 0 📌 0
We had a weekend full of group festivities! @pennchemistry.bsky.social
05.03.2025 13:10 — 👍 2 🔁 0 💬 1 📌 0 26.02.2025 19:35 — 👍 0 🔁 0 💬 0 📌 0
26.02.2025 19:35 — 👍 0 🔁 0 💬 0 📌 0

Congratulations to Dr. Christopher Sojdak on successfully defending his thesis! @pennchemistry.bsky.social
26.02.2025 19:34 — 👍 0 🔁 0 💬 1 📌 0
Congrats to the entire team: Chris, David, Hriday, Cass, Brandon, Pedro and Micah on their work out now in ACS Central Science! @pubs.acs.org @pennchemistry.bsky.social
26.02.2025 13:16 — 👍 1 🔁 0 💬 0 📌 0
Join us today for Chris Sojdak’s PhD thesis defense at 10 AM in Carolyn Hoff Lynch Lecture Hall! @pennchemistry.bsky.social
26.02.2025 13:07 — 👍 0 🔁 0 💬 0 📌 0
Welcome @pennchemistry.bsky.social prospective Fall 2025 students! Please visit our lab anytime during this weekend.
21.02.2025 22:34 — 👍 1 🔁 0 💬 0 📌 0