
A reaction scheme showing the direct alkoxycarbonylation of arenes using a dual ligand-based catalyst system.
A direct synthesis of versatile active esters! Massive congratulations to Simon Kaltenberger, Joshua Meinshausen, @jyotirmoydey.bsky.social and Celia Sanchez-Gonzalez on the development of a widely applicable sterically controlled alkoxycarbonylation of arenes now in JACS Au doi.org/10.1021/jacs...
02.12.2025 10:15 — 👍 3 🔁 2 💬 0 📌 0

Exttemely proud of this computational and experimental mechanistic work headed by @chimicafritz.bsky.social and supported by @monikaravi.bsky.social just out in @angewandtechemie.bsky.social
doi.org/10.1002/anie...
#chemsky
23.10.2025 16:25 — 👍 10 🔁 2 💬 2 📌 1
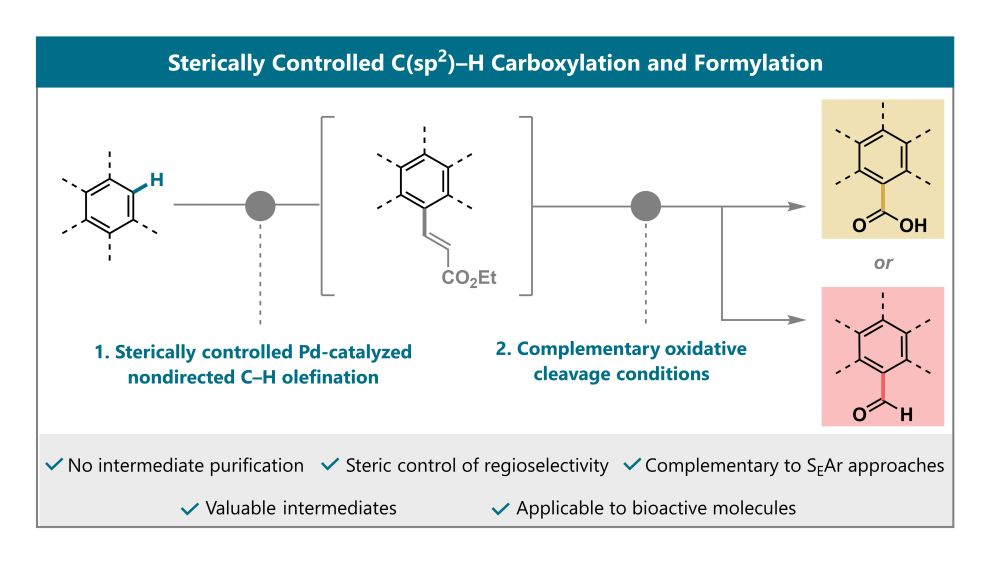
A reaction scheme showing a generic arene structure, which is first olefinated through a nondirected C-H olefination, followed by two oxidative cleavage pathways, one giving carboxylation products the other giving formylation products.
Access SEAr-type products in typically disfavored positions! Contratulatons to Rita, @jyotirmoydey.bsky.social and Elisa showin in Org. Lett. how nondirected C-H activation and oxidative cleavage can be used to obtain challenging regioisomers in formylation and carboxylation doi.org/10.1021/acs....
15.09.2025 10:26 — 👍 11 🔁 3 💬 0 📌 0

A simple, scalable method by Jurk & van Gemmeren @vangemmerenlab.bsky.social to convert secondary sulfonamides into sulfonyl fluorides using affordable #DAST ⚗️, yielding excellent results! These sulfonyl fluorides readily couple with amines to create diverse libraries.
#SuFEx
👉 buff.ly/hjkyEap
21.08.2025 07:00 — 👍 3 🔁 2 💬 0 📌 0
Thank you for featuring our work. It was indeed a great experience to publish with you!
14.07.2025 18:09 — 👍 1 🔁 0 💬 0 📌 0

Drei Nachwuchsforschende der Uni Kiel bei Nobelpreisträger-Tagungen
Die Einladungen sind auch eine Auszeichnung für die Chemie und die Wirtschaftswissenschaften an der CAU.
Congratulations to aol three @uni-kiel.de candidates who were selected to participate in the Lindau Nobel Laureate meeting. In particular of course our very own @sourjyamal.bsky.social. A well deserved recognition of his excellent doctoral studies. #proudPI #chemsky
www.uni-kiel.de/de/detailans...
13.07.2025 17:10 — 👍 8 🔁 1 💬 0 📌 0

Table of contents graphic showing the reaction of an aliphatic carboxylic acid with a styrene to give a γ-vinylidene lactone via tripple C-H functionalization. The structure of the ligand used is given as well as bullet points showing the main strenghts of the report, such as a broad substrate scope.
Great job Edis and @sourjyamal.bsky.social! Their work on the synthesis of γ-Alkylidene Lactones has just been accepted for publication in the @rsc.org journal @chemicalscience.rsc.org
doi.org/10.1039/D5SC...
04.07.2025 08:15 — 👍 13 🔁 4 💬 0 📌 0
Organic Letters :
Just saw on the OL homepage that the article currently leads the journals most read list, thanks for all the interest in our work! pubs.acs.org/action/showM...
29.06.2025 17:25 — 👍 2 🔁 1 💬 0 📌 0
Check out our newest C(sp³)–O bond formation strategy:
doi.org/10.1021/acs....
12.06.2025 09:18 — 👍 0 🔁 0 💬 0 📌 0

Graphical abstract of a manuscript detailing the reaction between a carboxylic acid and a styrene under palladium catalysis.
Congratulations to Edis and @sourjyamal.bsky.social, who have completed their studies on a new and efficient approach towards gamma-alkylidene lactones directly from carboxylic acids and lactones now posted on @chemrxiv.bsky.social
chemrxiv.org/engage/chemr... #chemsky #chemistry
11.04.2025 16:22 — 👍 14 🔁 4 💬 0 📌 0

A screebshot from the ACS Catalysis homepage shiwing the article in the most read list.
Thanks for all the interest in the study. It is now amongst the currently most read in ACS Catalysis
04.02.2025 05:48 — 👍 4 🔁 1 💬 0 📌 0
Thank you Fritz!
31.01.2025 17:54 — 👍 3 🔁 0 💬 0 📌 0
Smashing distal C–H bonds of the unprecedented β-non-quaternary acids has been a formidable task over the years; several years of our involvement in 'Ligand Design' finally broke the deadlock! Thanks to @vangemmerenlab.bsky.social for guiding us to achieve this feat !
31.01.2025 16:13 — 👍 7 🔁 0 💬 0 📌 0

We have developed a direct gamma-lactonization of carboxylic acids. The scope includes beta-non-quaternary subatrates that were until now out of reach for carboxylic acid gamma-C-H activation. Congratulations to Tianxiao and Sourjya. Have a look at the study now posted on ChemRXiv t.co/Wu0XtwsEQR
19.07.2024 06:11 — 👍 4 🔁 1 💬 0 📌 0
PhD student, looking for postdoc
Scientific Editor, RSC. Views my own.
Background in computational chemistry and education.
Sci-fi, blackgaze, queer horror books, MMOs - including an insistent love of Phantasy Star Online
Organic Synthesis Research Group at Indian Institute of Science (IISc) Bangalore
🔗 https://theharigroup.in/
Official home for the #Chemistry community #RealTimeChem (part of #ChemSky). Connecting chemists (on the old place since 2012. Curated by @doctorgalactic.bsky.social.
Join in by sharing chemistry using #RealTimeChem.
Graduate organic chemist in the Floreancig Lab at the University of Pittsburgh!! 🎉👩🔬#TeamBoron
Recovering prebiotic chemist. Editor in Chief at Cell Reports Physical Science @cp-cellrepphyssci.bsky.social. Personal account, views not my employer's, etc.
Professor of Chemistry 👨🔬 FU Berlin
https://www.bcp.fu-berlin.de/en/chemie/chemie/forschung/OrgChem/christmann/index.html
Loves Tool & learns Python #ChemSky
The Spectroscopy & Biocatalysis Group @ Uni Potsdam | DFG Heisenberg Fellow | Guest-Prof TU Berlin 2023/24 | Born at 338 ppm | Not a Strip Club
https://uni-potsdam.de/en/specbiocat | https://scholar.google.com/citations?user=ZtfyzRoAAAAJ&hl=en
Organic and organometallic PhD student 🇫🇷 at @didierlab.bsky.social at @tuda.bsky.social 🇩🇪
Assistant Professor and PI of the Sowden group a synthetic organic chemistry group at Auburn University.
Aussie living in the USA. https://sowdengroup.org
Postdoctoral researcher in the Ackermann group | Former PhD at U-Paris Cité w/ Dr. Farouk Berhal and Pr. Guillaume Prestat.
Catalysis & Heterocycles
https://www.ilv.uvsq.fr/catalyses-et-heterocycles
Pyridine destroyer
PhD at ICBMS Lyon | A. von Humboldt postdoctoral fellow MPI KoFo @cornellab.bsky.social | CNRS researcher Univ. Paris Saclay - Versailles ILV
Brit in Germany, nearing end of PhD with focus on lithium ion battery material development
Assistant Professor of Chemistry @UNC Chapel Hill
electrochemistry - materials - catalysis - energy storage
squarescheme.squarespace.com
Scientific Editor for Chemical Science, published by the RSC, and small-time journalist for Chemistry World. #ChemSky #ChemSci
Gaming, ballroom dancing, rollerskating, piano, and D&D - I’m acceptably mediocre at all of them! #Switch2
(he/him ♓️🏳️🌈)
CNRS researcher in Organic Chemistry at Aix Marseille University - France
Postdoctoral Researcher in Cooper Group @ University of Liverpool working in chemistry automation - Mostly making robots do chemistry so I don't have to.
Organic chemist, radiochemist, bioimaging scientist, living in Donostia-San Sebastián. Ex-UCBL & KU Leuven. Research associate @ #CIC-biomaGUNE interested in org chem and ion channels.
Science Correspondent @ChemistryWorld / DM or robinsonj@rsc.org / Best Writer (News) BSME Talent Awards 2023
Bioinorganic chemistry research group at the University of Rochester, USA. Studying bioinspired catalysis of energy-relevant reactions. Student-run account.











