
Regio- and Stereocontrolled-Synthesis of a Heterocycle
Fragment Collection Using Palladium Catalyzed C-H Arylation
Check out our latest work on from Sofia, Dani, Harry, Natalia and Matt on a C-H arylation approach to systematically access heterocycle fragments. / CEJ
doi.org/10.1002/chem...
12.11.2025 17:24 — 👍 1 🔁 0 💬 0 📌 0

Do Amino-Oxetanes Resemble Amides?
Check out our Matched Molecular Pairs study in
@chemrxiv.org comparing properties and structure.
Congrats @hikaruishikura.bsky.social and coworkers, in collaboration with Pfizer.
doi.org/10.26434/che...
02.09.2025 08:03 — 👍 8 🔁 5 💬 0 📌 0
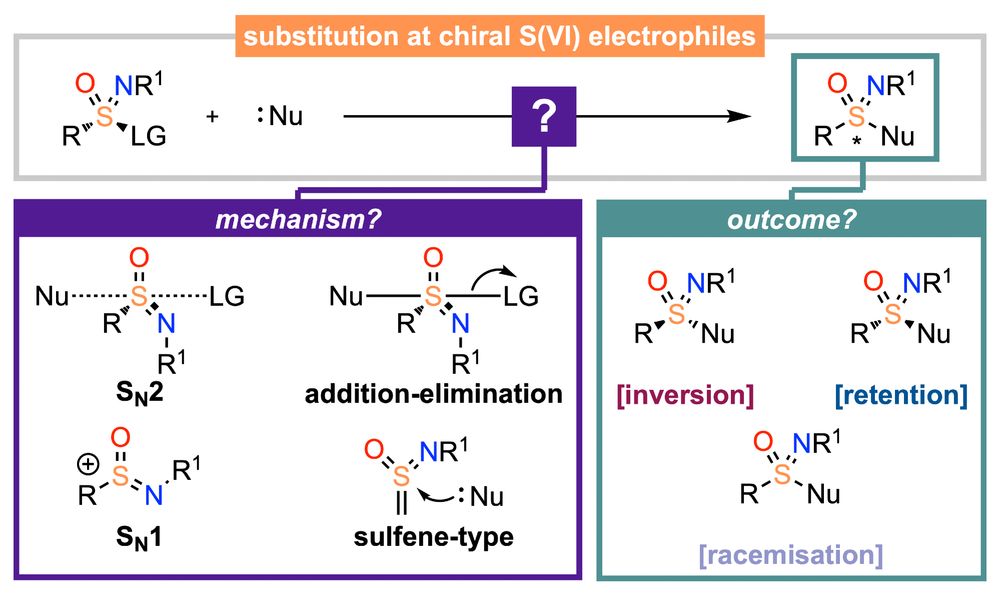
As asymmetric sulfur(VI) derivatives become more prevalent in the chemical sciences, especially in aza-derivatives, sulfoximines, sulfonimidamides, we have reviewed "The stereochemistry of substitution at S(VI)"
doi.org/10.1039/D5QO...
with Ollie Symes, @orgchemfront.rsc.org
02.09.2025 07:53 — 👍 6 🔁 2 💬 0 📌 0
SuFEx and SNAr on Sulfonimidoyl Fluorides - happy this is now published in ChemistryEurope.
chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/...
Catalytic SuFEx Reactivity of Sulfonimidoyl Fluorides with Functionalized Amines with Automation Applicable Conditions.
Congrats Nikki!
11.07.2025 08:52 — 👍 2 🔁 0 💬 0 📌 0
Happy to see our work on the divergent synthesis of difluorocyclobutanes, now published in #JOC: Synthesis of gem-Difluorocyclobutanes: Organolanthanum Enabled Synthesis and Divergent Catalytic Functionalization of gem-Difluorocyclobutanols. pubs.acs.org/doi/10.1021/... @hikaruishikura.bsky.social
11.07.2025 08:48 — 👍 4 🔁 2 💬 0 📌 0
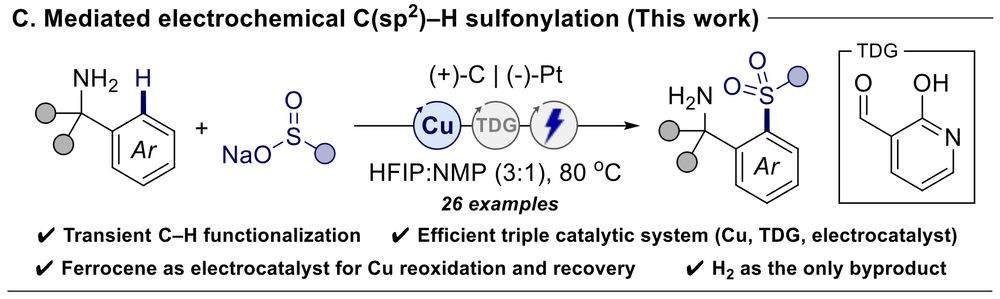
Electrochemical Copper Catalysis: A Triple Catalytic System for Transient C(sp2)–H Functionalization Through Mediated Electrolysis | @ChemRxiv.
Congrats Freeman!
doi.org/10.26434/che...
03.06.2025 12:53 — 👍 1 🔁 0 💬 0 📌 0
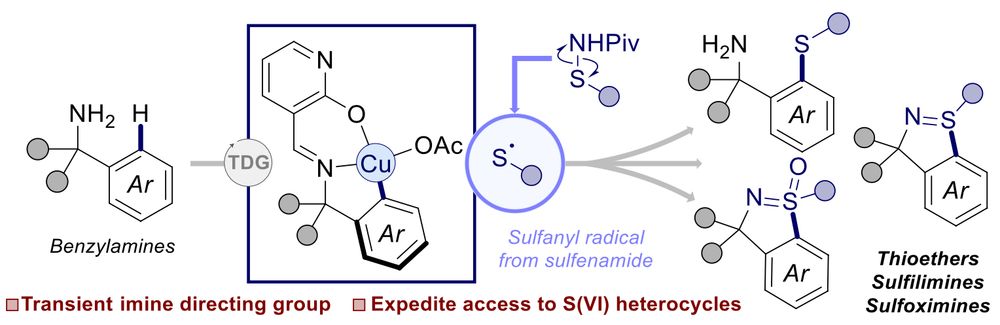
Congrats to Freeman and Peerawat on our recent @ChemRxiv - Synthesis of Cyclic Sulfilimines and Sulfoximines via Copper Mediated C(sp2)–H Sulfanylation of Benzylamines with a Catalytic Transient Directing Group.
chemrxiv.org/engage/chemr...
19.05.2025 09:54 — 👍 3 🔁 0 💬 1 📌 0
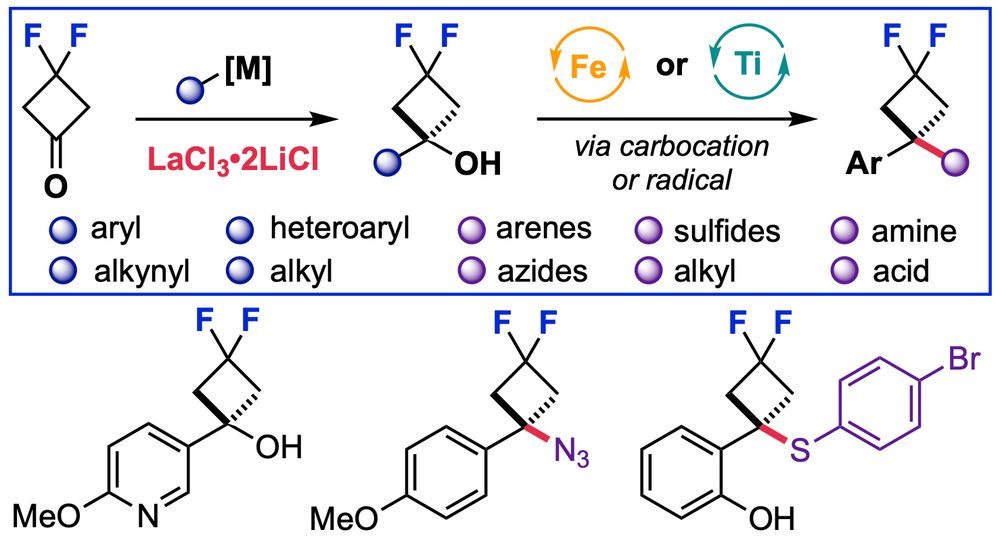
Interested in strained rings or fluorine for medicinal chemistry - check out Hikaru’s work on difluorocyclobutanes on ChemRxiv.
doi.org/10.26434/che...
19.05.2025 09:43 — 👍 6 🔁 2 💬 0 📌 1
Advanced Molecular Synthesis MRes | Study | Imperial College London
🌟 Unlock Your Potential in Advanced Molecular Synthesis!
Gain skills and knowledge to excel in the dynamic field of molecular synthesis.
🔗 Apply Today! www.imperial.ac.uk/study/course...
#Chemistry #MolecularSynthesis #ImperialCollege #Research #Innovation #PostgraduateStudies #MRes #Research
18.03.2025 11:18 — 👍 0 🔁 1 💬 0 📌 0

How to thrive at conferences
Five tips for making the most of meet-ups
🌟 Alumni Spotlight: Rupali Dabas 🌟 Celebrating the achievements of our MRes in Drug Discovery & Development alumni! Her article is in @chemistryworld.com on conferences. 🌍✨ 🔗 Read more: www.chemistryworld.com/careers/how-... #AlumniImpact #ChemistryCommunity #ImperialCollegeLondon #DrugDiscovery
03.04.2025 11:05 — 👍 2 🔁 2 💬 0 📌 0

New dimensions for covalent enantioprobe chemoproteomics at Imperial College London on FindAPhD.com
PhD Project - New dimensions for covalent enantioprobe chemoproteomics at Imperial College London, listed on FindAPhD.com
We are advertising a 2nd fully funded PhD studentship with Ed Tate and ICB CDT for October:
New dimensions for covalent enantioprobe chemoproteomics
#chemicalbiology, #synthesis, #chemoproteomics, #CHfunctionalisation.
www.findaphd.com/phds/project...
21.03.2025 12:13 — 👍 1 🔁 2 💬 0 📌 0

Studentships for October 2025 entry
2025 Studentship Opportunities
We are advertising an exciting PhD studentship related to covalent drug discovery with Tate, Armstrong & Mann groups and Vertex pharmaceuticals with ICB CDT.
"Warheads take the strain in chemoproteomics"
#Synthesis, #Strained_rings, #Chemoproteomics
Apply here: www.imperial.ac.uk/chemical-bio...
11.03.2025 09:45 — 👍 2 🔁 2 💬 0 📌 0
Description
Please note that job descriptions are not exhaustive, and you may be asked to take on additional duties that align with the key responsibilities ment...
We are recruiting for a postdoctoral Research Associate in Synthetic Chemistry to work in C-H functionalisation. Come join us Imperial! @imperialchemistry.bsky.social
3-year position. Closing: 19-March-2025. shorturl.at/uwERp
#Chempostdoc, #CHfunctionalization, #Heterocycles
19.02.2025 13:39 — 👍 12 🔁 17 💬 0 📌 0
Thanks so much to @jamesabull.bsky.social and team for this great review, online now and #OpenAccess! @imperialchemistry.bsky.social
19.02.2025 11:29 — 👍 4 🔁 2 💬 0 📌 0
RSC Organic Chemistry Community South and East Regional Meeting 2025
The RSC Organic Chemistry Community South-East Regional Meeting will be hosted by Imperial Chemistry
@imperialchemistry.bsky.social on Friday 28th March 2025 with an excellent speaker line-up. For more details, to register, & submit poster abstract (PhD/PDRAs), see here: www.rsc.org/events/detai...
17.02.2025 16:15 — 👍 4 🔁 3 💬 0 📌 0

Celebrating Christmas with a new cover design inspired by @jamesabull.bsky.social's team and their work on sulfoximine-bicyclo[1.1.0]butanes. These warheads are a game-changer for protein research with their tuneable reactivity!
#sciart ⌬ #research ⌬ #chemistry
23.12.2024 09:48 — 👍 3 🔁 1 💬 1 📌 0

Bull Group at table ahead of a meal on a lab Christmas party
Good times at the group Christmas party
17.12.2024 00:09 — 👍 3 🔁 0 💬 0 📌 0
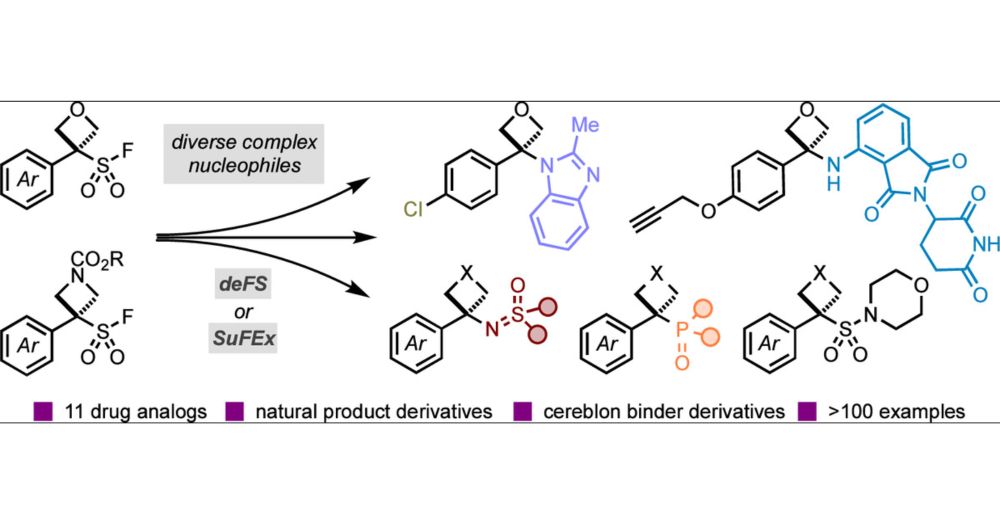
Harnessing Oxetane and Azetidine Sulfonyl Fluorides for Opportunities in Drug Discovery
Four-membered heterocycles such as oxetanes and azetidines represent attractive and emergent design options in medicinal chemistry due to their small and polar nature and potential to significantly impact the physiochemical properties of drug molecules. The challenging preparation of these derivatives, especially in a divergent manner, has severely limited their combination with other medicinally and biologically important groups. Consequently, there is a substantial demand for mild and effective synthetic strategies to access new oxetane and azetidine derivatives and molecular scaffolds. Here, we report the development and use of oxetane sulfonyl fluorides (OSFs) and azetidine sulfonyl fluorides (ASFs), which behave as precursors to carbocations in an unusual defluorosulfonylation reaction pathway (deFS). The small-ring sulfonyl fluorides are activated under mild thermal conditions (60 °C), and the generated reactive intermediates couple with a broad range of nucleophiles. Oxetane and azetidine heterocyclic, -sulfoximine, and -phosphonate derivatives are prepared, several of which do not have comparable carbonyl analogs, providing new chemical motifs and design elements for drug discovery. Alternatively, a SuFEx pathway under anionic conditions accesses oxetane-sulfur(VI) derivatives. We demonstrate the synthetic utility of novel OSF and ASF reagents through the synthesis of 11 drug analogs, showcasing their potential for subsequent diversification and facile inclusion into medicinal chemistry programs. Moreover, we propose the application of the OSF and ASF reagents as linker motifs and demonstrate the incorporation of pendant groups suitable for common conjugation reactions. Productive deFS reactions with E3 ligase recruiters such as pomalidomide and related derivatives provide new degrader motifs and potential PROTAC linkers.
Very happy to use my first post here to say thanks and congratulations to the team for their great work on our latest study on oxetanes and azetidines, out now in JACS. doi.org/10.1021/jacs...
16.12.2024 23:45 — 👍 12 🔁 2 💬 1 📌 0
Prof of Supramolecular and Materials Chemistry at University of Glasgow. Chair of RSC Porous Materials Interest Group. Pores and snores in equal measure. Personal account with occasional boasting.
Bren Professor of Chemistry, Norman Davidson Leadership Chair, Caltech
http://reismangroup.caltech.edu/
Robert Paton | Fixman-Ladanyi Professor of Chemistry at Colorado State University | #compchem | http://linktr.ee/patonlab | 🇬🇧🇺🇸
Chemistry professor at CMU. Connecting chemical sciences with AI #MachineLearning and automated experimentation. #tarheels fan. Care: #design, #photography #Ukraine #cats🐈 Rants are mine
Using quantitative, data-driven approaches to make synthetic chemistry more predictable. Phase II CCI #NSFFunded
🌐 https://ccas.nd.edu/
📷 https://www.instagram.com/nsf_ccas/
Lecturer in Chemistry at Imperial College London | Uses computers to simulate electrons
Royal Society University Research Fellow at the University of St Andrews
https://craigpj2.wixsite.com/johnstonlab
The Bsky account for the UKRI funded Foldamer Network: foldnet.uk
The UK Catalysis Hub is a consortia of universities involved in catalysis research. It brings together forty-six universities, fifteen companies, the STFC Harwell science facilities and thirteen international partners.
https://www.ukcatalysishub.co.uk
Organic chemist, currently postdoc in the Shenvi lab at Scripps Research.
Angewandte Chemie, a journal of the German Chemical Society (@gdch.de), is a leading journal in #chemistry published by Wiley-VCH (@wiley.com) in Weinheim, Germany
https://onlinelibrary.wiley.com/journal/15213773
Research associate in the Crimmin group at Imperial College.
Research group of Organic Chemistry at the University of Bari. Expertise in: Flow technology, Photocatalysis, Synthetic Methodologies, Green and Sustainable Development.
Visit: https://www.renzoluisi-lab.com/
Rapid publication of organic synthesis, physical organic, chemical biology and supramolecular chemistry research.
Published by @rsc.org 🌐 Website: rsc.li/orgbiomolchem
Institute of Chemical Biology, Centre for Doctoral Training, ICB-CDT, Imperial College London, Chemistry
Student-run account of the Baudoin research group at the University of Basel
The first journal dedicated to the study of mechanochemistry, from fundamental to applied innovations.
Published by @rsc.org 🌐 Website: rsc.li/RSCMechanochemistry
ETOC is an online symposium about how technological innovation is transforming organic chemistry. 29-30 April 2026
Associate Professor @UMN. Urgently spreading and learning the OChem Gospel. As a researcher/mentor, I want to (i.) address synthetic challenges associated with CNS disorders and (ii.) invent new reactions of general use to drug discovery.


















