
Our CRTI group PROs and Poop! 💩 (intersection of patient-reported outcomes and fecal microbiome) #ASHCRTI cc @ash.hematology.org @biostatgirl.bsky.social
05.08.2025 22:45 — 👍 3 🔁 1 💬 1 📌 0@biostatgirl.bsky.social
Oncology & Patient-Reported Outcomes Biostatistician looking for a new social media home. I love bar charts too. Opinions are my own. https://duecklab.github.io

Our CRTI group PROs and Poop! 💩 (intersection of patient-reported outcomes and fecal microbiome) #ASHCRTI cc @ash.hematology.org @biostatgirl.bsky.social
05.08.2025 22:45 — 👍 3 🔁 1 💬 1 📌 0

Currently at #EHA2025: @broeckelmann.bsky.social presenting our recent @thelancethaem.bsky.social commission on adverse events, starting with our paper (with @biostatgirl.bsky.social) on advancing the measurement & dissemination of AE data HT @eddiecliff.bsky.social #EHA25
13.06.2025 13:36 — 👍 4 🔁 2 💬 1 📌 1If you want to hear more from the authors of the first set of commission manuscripts, our @thelancethaem.bsky.social podcast is now online! www.buzzsprout.com/1564352/epis... ft @biostatgirl.bsky.social @broeckelmann.bsky.social #EHA2025 #EHA25
13.06.2025 13:48 — 👍 2 🔁 2 💬 0 📌 0
Presenting a #patientreportedoutcomes workshop at Society for Clinical Trials 46th Annual Meeting! #SCT2025 #clinicaltrials #PROs @christinayap.bsky.social
18.05.2025 22:29 — 👍 3 🔁 0 💬 0 📌 1
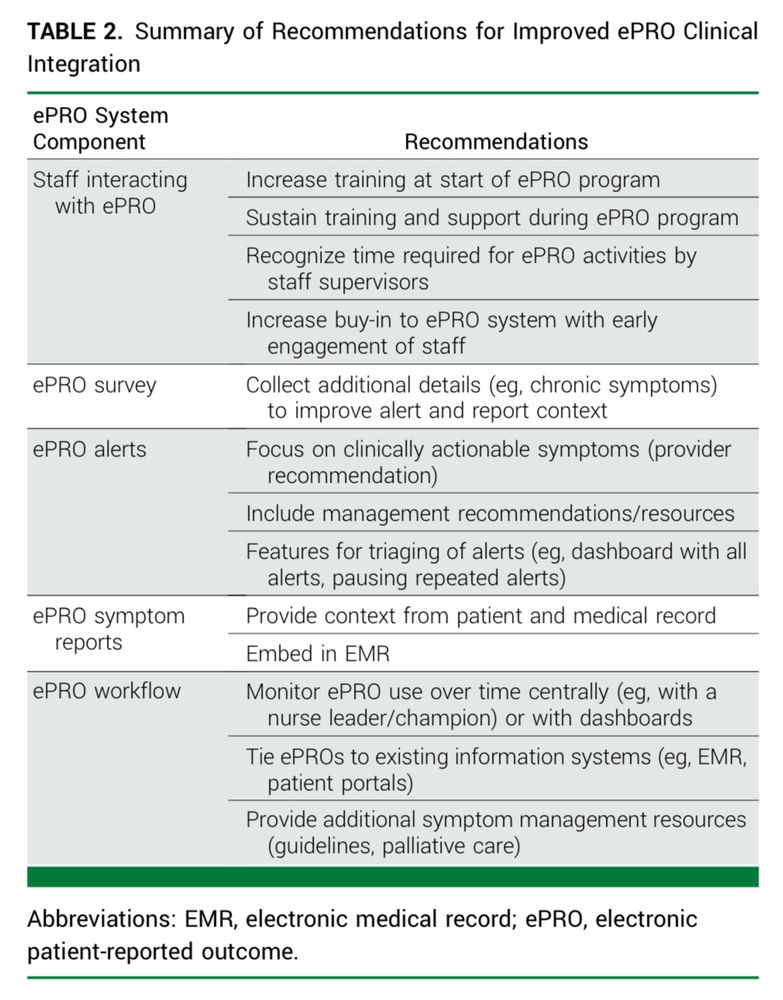
Implementation of Symptom Monitoring With Electronic Patient-Reported Outcomes: Perspectives and Recommendations From Community Oncology Practices (Alliance AFT-39).
ascopubs.org/doi/10.1200/...
@pallonccop.bsky.social
#PallOnc #CancerCare
@ethanbasch.bsky.social
@biostatgirl.bsky.social




What an incredible week in Casablanca, Morocco, for @ash.hematology.org Clinical Research Training Institute & Training Day, Mediterranean, Middle East, and Northern Africa. I’m so inspired by the growth of the trainees this week & I’m excited to see the future #hematology leaders they become! #CRTI
05.04.2025 12:24 — 👍 2 🔁 0 💬 0 📌 0
PRO-TECT trial of ePROs final results @biostatgirl.bsky.social
- 52 onc practices, randomized to weekly ePRO symptom surveys vs usual care
- no difference in OS
- 6% reduction in ER visits
- delayed deterioration of HRQOL w/ ePROs
Important for patients & health care system. #oncsky
buff.ly/3Xi7cen
🚨Heme #MedEd enthusiasts!🚨
Want to level⬆️ heme education skills? 🩸ASH MEI is accepting applications!
✅ Expert yr-long mentoring
✅ Develop leaders in heme MedEd scholarship
✅ Community of like-minded educators
It was game-changer in my career- as participant, now faculty & co-director! Join us!

Our invited review on how to integrate patient-reported outcomes into hematology clinical trials to measure treatment tolerability. Many thanks to Drs. Thanarajasingam and @biostatgirl.bsky.social for your collaboration! #medsky #lymsm #leusm #mmsm pubmed.ncbi.nlm.nih.gov/39198102/
17.02.2025 15:13 — 👍 5 🔁 1 💬 0 📌 0
Onsite retreat today - my fellow biostatisticians spelling out MAYO!
11.02.2025 21:00 — 👍 3 🔁 0 💬 0 📌 0
The fearless leaders of #SISAQOL: Anders Ingelgaard & Madeline Pe — thank you for your vision and work to enhance PRO statistical analysis for everyone!
04.02.2025 14:50 — 👍 1 🔁 0 💬 0 📌 0
Stakeholder panel at #SISAQOL. Interesting discussion about whether collection of PROs post-progression is feasible, how to make PROs less burdensome, & where to place PROs in the hierarchy of clinical trial endpoints. Universal agreement that SISAQOL moves the field forward!
04.02.2025 14:36 — 👍 2 🔁 2 💬 0 📌 0


Coming soon from #SISAQOL: guidebook, interactive table, glossary, patient material, and more! Will be posted on the SISAQOL website. www.sisaqol-imi.org
04.02.2025 14:34 — 👍 2 🔁 2 💬 0 📌 0
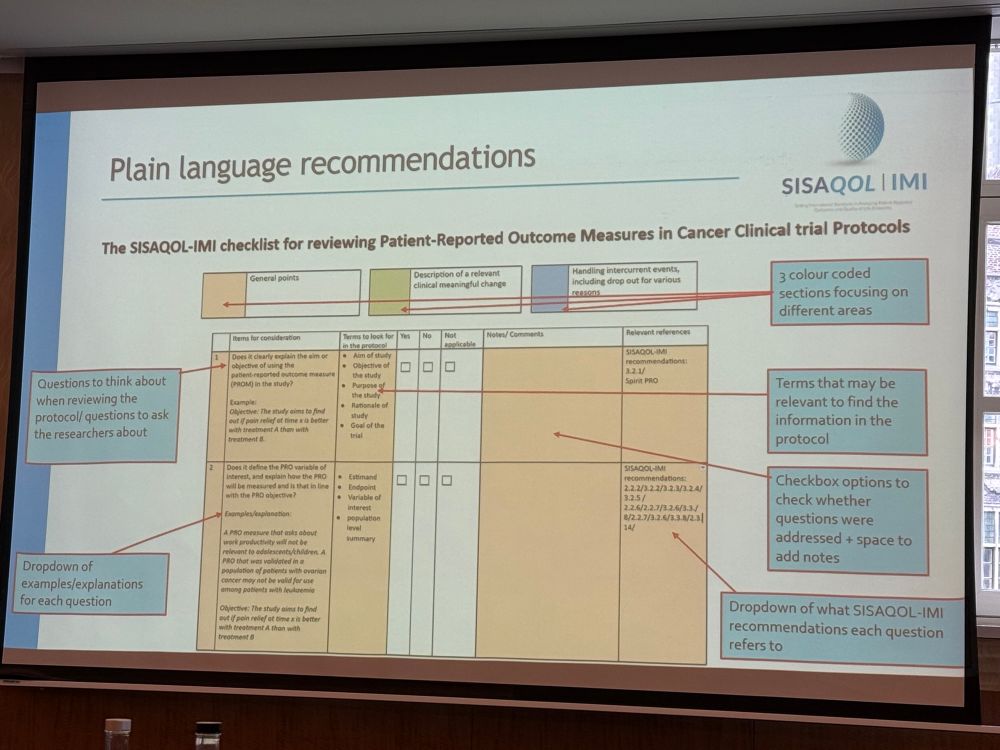

Coming soon from #SISAQOL for patient advocates & patient research partners: checklist for reviewing PRO measures in protocols, and checklist for reviewing visualizations in cancer clinical trials.
04.02.2025 11:19 — 👍 5 🔁 2 💬 0 📌 0


#SISAQOL developed recommendations on how to select a threshold for interpreting PROs and how to report that threshold.
04.02.2025 10:19 — 👍 4 🔁 2 💬 0 📌 0

Harmonized terms to replace the vague term “MID” from #SISAQOL! Need to be clear about whether you are talking about within-patient change (eg, responder analysis) or three group-level options (mean comparisons within or between groups - see slides).
04.02.2025 10:14 — 👍 3 🔁 2 💬 0 📌 0

Last two #SISAQOL #datadiz recommendations: indicate the directionality of PRO scores (what is good/bad, or what is improved/worsened) and use asterisks instead of p-values in graphs for audiences outside of the scientific community.
04.02.2025 10:14 — 👍 4 🔁 2 💬 0 📌 0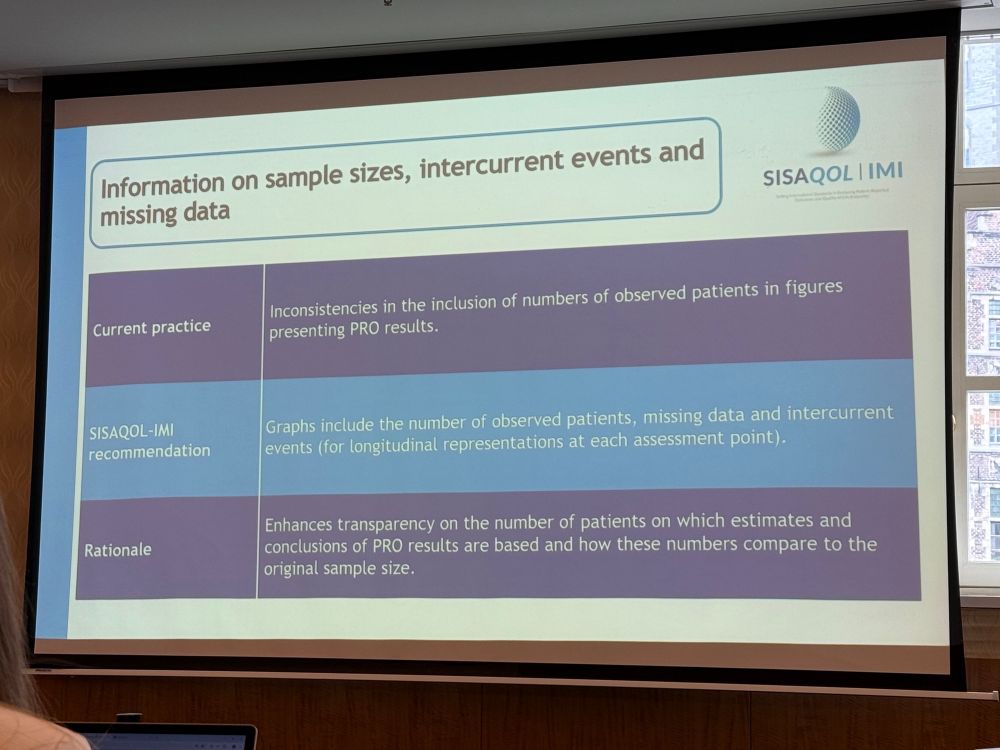

This #SISAQOL #dataviz recommendation gets a dedicated post. While I agree in general with including sample size, missing data, & incurrent event information on all graphs for clarity, three lines of text per arm may be infeasible for multi-arm trials and may be more readable in a standalone table.
04.02.2025 10:13 — 👍 2 🔁 2 💬 0 📌 0

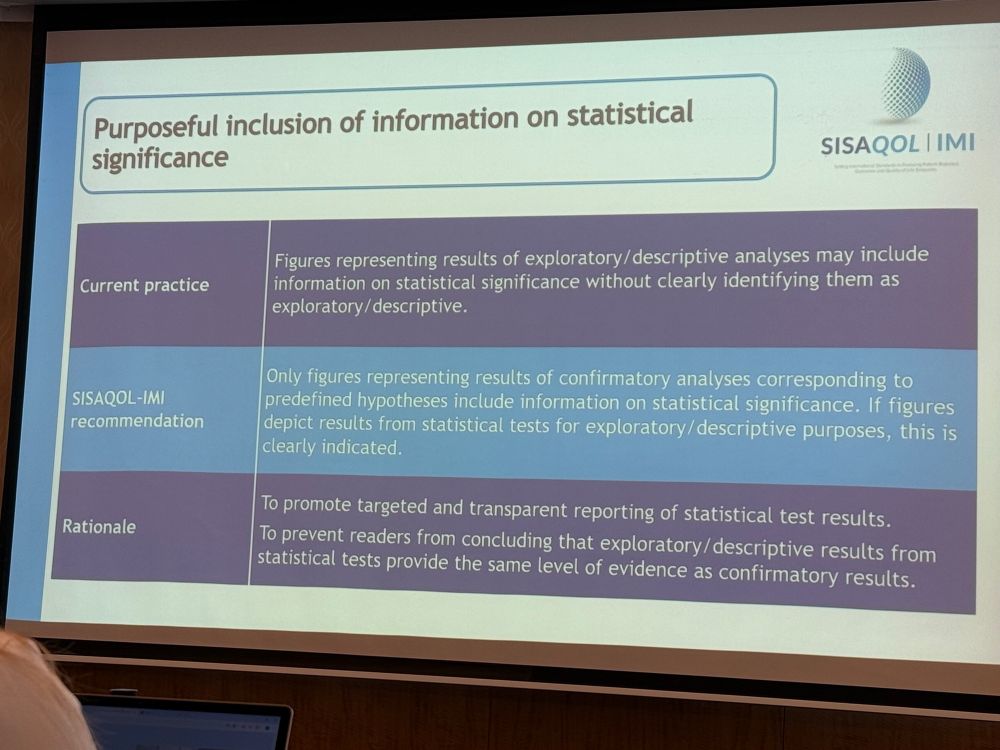

Excited that #SISAQOL included recommendations about #dataviz for #patientreportedoutcomes! Graphs vary by audience consistent with prior literature. Include p-values for preplanned tests only, and use consistent y-axis scaling throughout your paper.
04.02.2025 10:11 — 👍 2 🔁 2 💬 0 📌 0



Next #SISAQOL turns to #patientreportedoutcomes in single-arm trials. PRO objectives should be clearly defined & can be descriptive (more common) or comparative to historical control data. Important to consider missing data & incurrent events as in randomized trials.
04.02.2025 09:21 — 👍 2 🔁 2 💬 0 📌 0
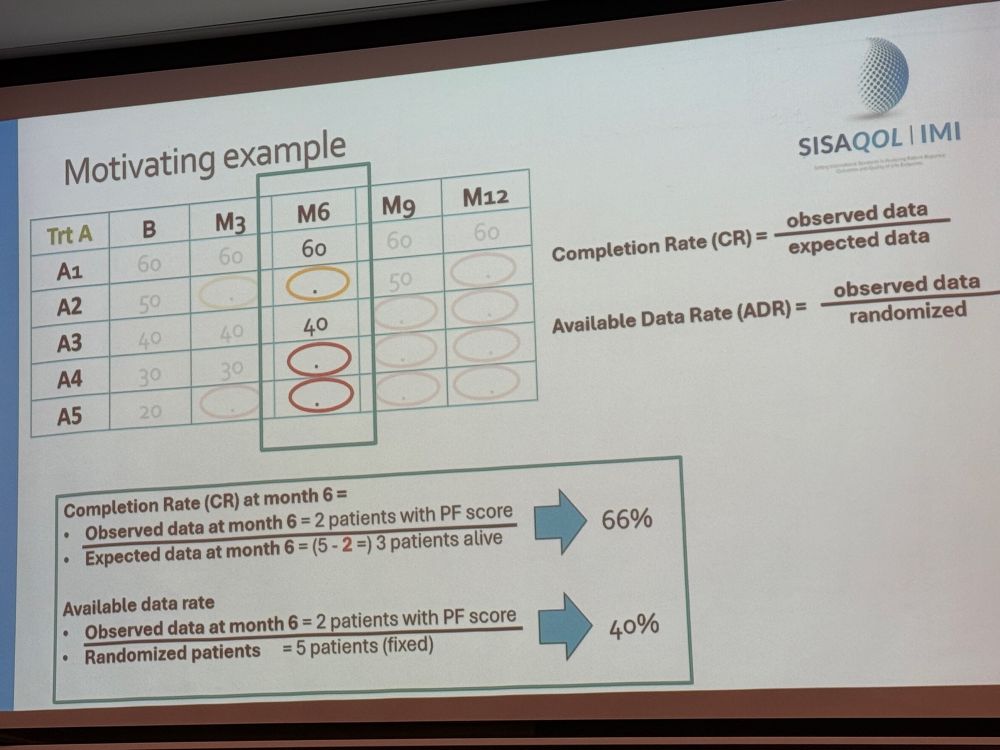


Next #SISAQOL highlights involve missing data. Need to separate missing data from intercurrent events, and recommendations reinforce using “completion rate” & “available data rate” - quit using “compliance rate” in your papers! Finally, avoid biased missing data approaches.
04.02.2025 09:19 — 👍 2 🔁 2 💬 0 📌 0

Diving into #SISAQOL recommendations. First highlights involve handling death or other intercurrent events. Basically there is no standard approach but need to be clear in the approach that you are using. Some new language here that many folks might not be familiar with…
04.02.2025 09:18 — 👍 2 🔁 2 💬 0 📌 0

Excited for a full day of new #SISAQOL recommendations for the statistical analysis of #patientreportedoutcomes in cancer clinical trials!
04.02.2025 08:32 — 👍 4 🔁 2 💬 0 📌 0Please select the one emoji that best represents your feeling towards our research paper developing emoji scales for assessing #patientreportedoutcomes: 😩 ☹️ 😐 🙂 😄
08.01.2025 18:13 — 👍 1 🔁 0 💬 1 📌 0Excited to see #PROCTCAE and #GP5 in the inavolisib label! Recurring points in the FDA discussion about needing dose optimization in early drug development to lower doses, maintain efficacy, and improve tolerability. Also comments about needing enrollment of diverse patients. #SABCS24
10.12.2024 19:21 — 👍 2 🔁 3 💬 0 📌 0
FDA panel on recent drug approvals in breast cancer at #SABCS24. Comparison of adjuvant CDK4/6 inhibitor trials, and lots of discussion about need for better dose optimization earlier in drug development.
10.12.2024 19:17 — 👍 2 🔁 1 💬 0 📌 0Excited to share statistical design aspects that can improve patient experience! See everyone in San Antonio soon! #SABCS24
09.12.2024 19:22 — 👍 8 🔁 2 💬 0 📌 0
First ever statistician meet up at #ASH24! Appreciate that @ash-hematology.bsky.social is working to foster broader statistician engagement in the annual meeting and the broader hematology field. @susanmgeyer.bsky.social
08.12.2024 00:25 — 👍 2 🔁 0 💬 0 📌 0



Free multiple myeloma response criteria from the pee! Excellent #ASH24 presentation by @rahulbanerjeemd.bsky.social showing that removing 24-hr urine changes response for only 1.1% of 645 patients in the BMT CTN 0702 clinical trial.
07.12.2024 18:20 — 👍 6 🔁 1 💬 0 📌 1
Check out all of this year's #MajorPROsASH, the top 20 best patient-reported outcomes & quality of life abstracts from #ASH24. 🧵 #ASHEducation #ASHKudos cc @ash-hematology.bsky.social
04.12.2024 17:35 — 👍 10 🔁 3 💬 0 📌 1