
How great leaders stand out.
24.07.2025 21:11 — 👍 0 🔁 0 💬 0 📌 0@senglaitan.bsky.social
Drug Hunter/Developer & Biotech Executive | Chief Scientific Officer at TFC (The First Cell) Therapeutics

How great leaders stand out.
24.07.2025 21:11 — 👍 0 🔁 0 💬 0 📌 0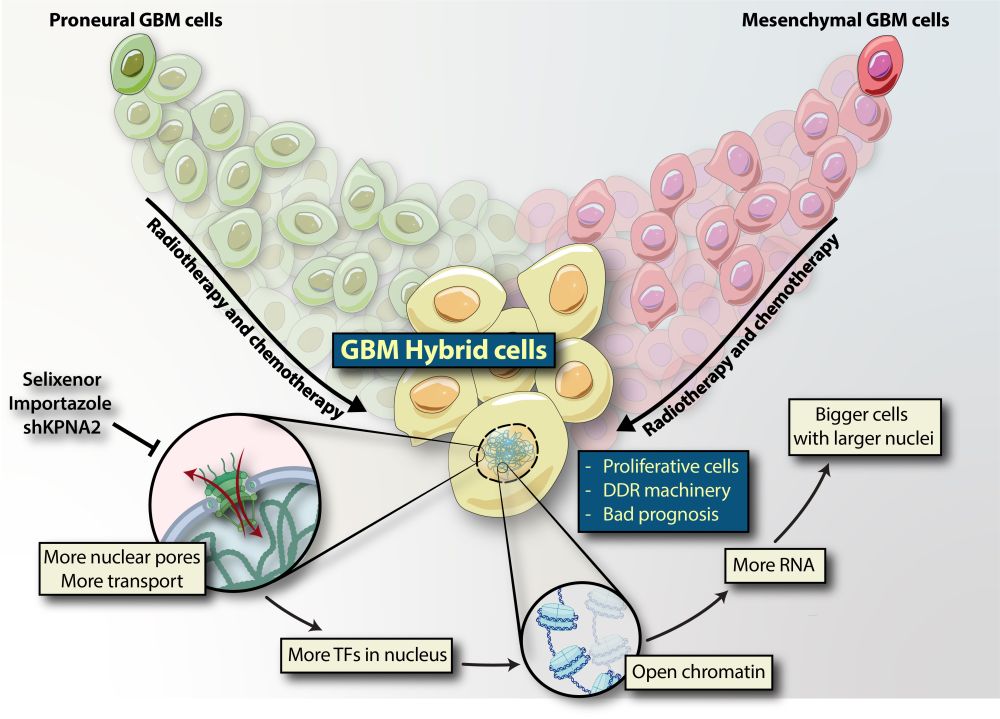
Background: Despite extensive research efforts, glioblastoma (GBM) remains a deadly disease with poor prognosis. Although previous studies have identified various cell states within GBM tumors, the molecular mechanism underlying adaptive GBM cell plasticity induced by conventional therapy remains unclear. Methods: We used fluorescent reporters for proneural (PN) and mesenchymal (MES) subtypes to monitor GBM cell plasticity in real-time across multiple patient-derived cell lines. This approach revealed cells that concurrently expressed both proneural and mesenchymal markers. To investigate this unique hybrid population, we implemented a comprehensive methodological approach encompassing bulk and single-cell RNA sequencing, single-cell ChIP sequencing, nuclear proteomics, high-resolution imaging, orthotopic mouse models, clinical dataset analysis, and pharmacological and genetic techniques. This multifaceted strategy allowed us to gain functional and molecular insights into this distinct cellular population. Results: We showed that these hybrid cells are increased by conventional therapies, and are resistant to these therapies. At the molecular level, hybrid cells display significant alterations in chromatin structure and nuclear protein composition, elevated transcriptional activity, Myc activation, and improved transport between the nucleus and cytoplasm. Genetic and pharmaceutical inhibition of the nuclear import/export shuttling machinery, increased in hybrid cells, effectively suppressed adaptive GBM cell plasticity and hybrid identity, thereby enhancing the sensitivity of GBM cells to therapies. Conclusion: Our results indicate that GBM hybrid cells play a crucial role in chemoradiation resistance. The nuclear transport machinery presents a potential therapeutic target for hybrid cells, offering a way to counteract the typical resistance to treatment observed in GBM.
🧠 Why does glioblastoma always outsmart treatment? In our #Neuro-Oncology paper, we identified proneural-mesenchymal hybrid glioblastoma cells that are resistant to therapy and dependent on nuclear import. doi.org/10.1093/neuo...
Short walkthrough below. Let’s dive in! 🧵 (1/9)
#GBM, #BrainTumor
Thrilled to share our latest review on the treatment of systemic lupus.
Here is the open-access read : rdcu.be/ewD2J

A cheatsheet for spotting a toxic leader, by George Stern.
13.07.2025 22:51 — 👍 0 🔁 0 💬 0 📌 0
Great to see this paper, a long-time collaboration with Betsy Barnes' lab, is finally published, "TLR-induced STK25 activation promotes IRF5-mediated inflammation" doi.org/10.26508/lsa...
#OpenAccess #lupus #SLE @lsajournal.org