Outstanding work!
17.08.2025 10:48 — 👍 3 🔁 0 💬 0 📌 0
Beautiful structure!
17.08.2025 10:47 — 👍 0 🔁 0 💬 0 📌 0
Absolutely magnificent lecture. Insight on how to properly apply computations to polar organometallics!
10.07.2025 10:59 — 👍 4 🔁 2 💬 0 📌 0

Opening the conference #EuCOMC2025 @laurelschafer.bsky.social on the intricacies of #hydroaminoalkylation chemistry
06.07.2025 15:05 — 👍 21 🔁 6 💬 0 📌 1


Stunning second #plenary talk #eucomc2025 by #DannyBroere @utrechtuniversity.bsky.social on the power of #bimetallic #cooperation in #catalysis
07.07.2025 08:02 — 👍 18 🔁 4 💬 0 📌 0

Today at the age of 86 my PhD supervisor Prof. Dr. Alexander Pozharskii passed away.
Rest in peace dear friend, thank you for everything, your work will not be forgotten, and your legacy will live on forever in your students, and in the students of your students.
10.05.2025 12:28 — 👍 2 🔁 0 💬 0 📌 0
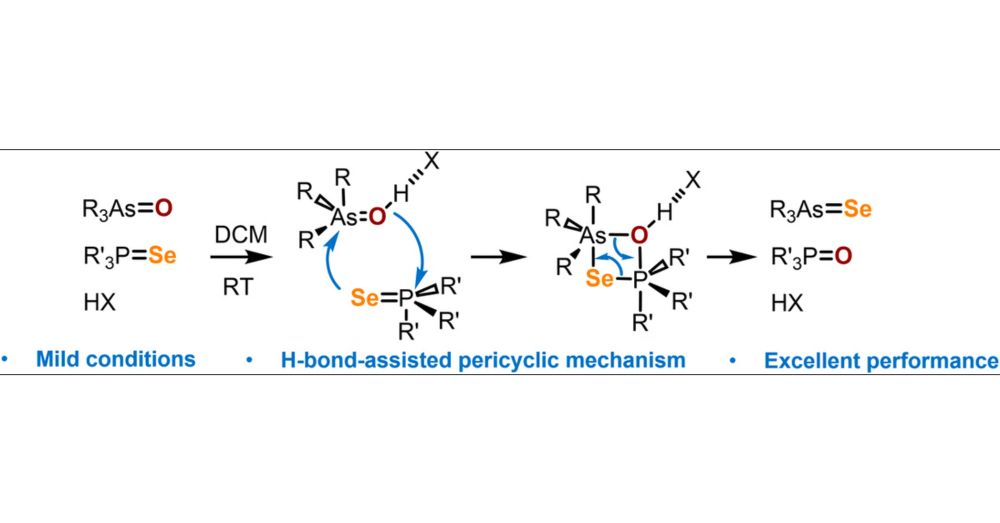
Hydrogen-Bond-Assisted Chalcogen Transfer between Phosphine Selenides and Arsine Oxides
The Brønsted acid catalysis is widely regarded as one of the most effective methods for activating inert substrates and enabling unique reactivity. In this work, we introduce the first example of H-bond-assisted chalcogen exchange between arsine oxides and phosphine selenides under mild conditions, providing a powerful approach to the synthesis of arsine selenides. The reaction proceeds successfully in both protic and aprotic solvents and is accelerated by the presence of any nonaqueous acid. This newly discovered reaction is tested for various arsine oxides R3AsO (R = Ph, Et, nBu, iPr) and phosphine selenides R3PSe (R = Ph, Me, Et, tBu) and overall demonstrates high conversion, although the use of reagents with bulky substituents significantly hinders its efficiency. The reaction mechanism involves the formation of a four-membered cyclic transition state, which requires overcoming steric and electrostatic repulsion, as well as significant distortion of the reagents’ tetrahedral geometry. Hydrogen bonding with the As═O fragment helps to reduce electrostatic repulsion between the P═Se and As═O groups, making the formation of the cyclic intermediate more favorable.
Check our new paper in @pubs.acs.org Inorganic Chemistry on how hydrogen bonding facilitates chalcogen exchange between arsine oxides and phosphine selenides.
pubs.acs.org/doi/10.1021/...
in Memoriam Prof. Dr. Lina M. Epstein (A.N.Nesmeyanov Institute of Organoelement Compounds RAS)
06.05.2025 08:25 — 👍 3 🔁 1 💬 0 📌 0
Very happy to have the opportunity to share our research at the Chemiedozententagung 2025
18.03.2025 14:35 — 👍 3 🔁 0 💬 0 📌 0
Join us in Bern this summer in @eucomc2025.bsky.social it is going to be so much fun!👇#EUCOMC2025
06.02.2025 05:59 — 👍 20 🔁 5 💬 0 📌 0
Can't wait to attend this wonderful meeting of organometallic chemists! Have you already registered? If not, it is time to be an early bird!
11.01.2025 17:28 — 👍 5 🔁 3 💬 0 📌 0
Please add me as well
28.11.2024 08:51 — 👍 2 🔁 0 💬 0 📌 0
Cooperative effects/reactivities and stimuli-responsive main-group systems. 2*father, TT Ass. Prof. at Uni Regensburg, #newPI #FirstGenAcademic #Humboldtian he/him
Web: https://go.ur.de/schorpp-group
Instagram: @schorpplab
A career network featuring science jobs in academia and industry.
Visit our platform at www.science.hr
Passionate about homogeneous catalysis, ligand design, and organometallic chemistry; can’t spend enough time in the mountains
Organometallic Main Group Metal Chemistry, mainly s- and p-block but nowadays also some d- and f-block. @FAU Erlangen
Passionate organic chemist and sulfur enthusiast. Assistant professor at the University of Regensburg.
Maingroup Chemistry | Postdoc @Mulveys Group | Strathclyde Glasgow
Managing Editor (he/him/his) Beilstein Journal of Organic Chemistry #BJOC • Completely free for authors + readers • Quality over profit • @beilstein-institut.bsky.social
Interested in 🧪🍲🏝️
...also a professional smalltalker
Scientific Editor for Chemical Science, published by the RSC, and small-time journalist for Chemistry World. #ChemSky #ChemSci
Gaming, ballroom dancing, rollerskating, piano, and D&D - I’m acceptably mediocre at all of them! #Switch2
(he/him ♓️🏳️🌈)
Inorganic, organometallic and bioinorganic chemistry updates from Dalton Transactions.
Published by @rsc.org 🌐 Website: rsc.li/daltontrans
Flagship journal for @rsc.org, open access with no publication fee, all topics in chemistry. chemicalscience-rsc@rsc.org
Celebrating our 15th year in 2025! 🌐 Website: rsc.li/chemscience
Die DFG ist die größte Forschungsförderorganisation und die Selbstverwaltungsorganisation der Wissenschaft in Deutschland.
www.dfg.de
Impressum: https://www.dfg.de/de/service/kontakt/impressum
Social Media-Netiquette: https://www.dfg.de/de/service/presse
Student-run twitter account for the Hartwig Group at UC Berkeley. 🧪
https://hartwig.cchem.berkeley.edu/
PhD student in the Zipse Group at LMU Munich, specializing in computational chemistry, primarily focused on organic systems.
Offizieller Kanal der Gesellschaft Deutscher Chemiker (GDCh). Hier schreibt die GDCh-Öffentlichkeitsarbeit. #chemsky http://gdch.de/impressum.html
Channel of the German Chemical Society (GDCh)
Professor of Inorganic Chemistry University of Strathclyde Glasgow Scotland
XXVI European Conference on Organometallic Chemistry
July 6-10, 2025, University of Bern, Switzerland #EUCOMC2025
https://eucomc2025.scg.ch
Graduate Researcher in Computational Chemistry @grimmelab.bsky.social
| M. Sc. @rwth.bsky.social and with M. Head-Gordon @ucberkeleyofficial.bsky.social
Development and application of efficient computational chemistry methods - based @unibonn.bsky.social.
This account is managed by group members of Prof. Stefan Grimme.
Angewandte Chemie, a journal of the German Chemical Society (@gdch.de), is a leading journal in #chemistry published by Wiley-VCH (@wiley.com) in Weinheim, Germany
https://onlinelibrary.wiley.com/journal/15213773














