Congrats!
19.06.2025 01:05 — 👍 1 🔁 0 💬 1 📌 0Congrats!
19.06.2025 01:05 — 👍 1 🔁 0 💬 1 📌 0Thanks a lot! 😁
02.04.2025 14:19 — 👍 0 🔁 0 💬 0 📌 0Lovely to see my paper from @natureneuro.bsky.social 😀
18.03.2025 14:02 — 👍 3 🔁 0 💬 1 📌 0
8/n
🔥 Since nAChR activation prevents subsequent DA axon depolarisation, we need to rethink DA function models based on somatic firing - not all axons will respond to action potentials recorded at the cell body.
7/n
🔑 Conclusion: depression of DA release is the dominant effect at lower nAChR activation levels, although ChIs may be able to trigger DA release in some cases (enough active nAChRs, high synchrony of ChIs actions).
6/n
🧠 A computational model predicted:
1️⃣ Fluctuating ChI activity → inverse DA deviation
2️⃣ Multiphasic ChI activity → depression of phasic DA release during learning
5/n
🐭 In vivo, inhibiting nAChRs enhanced striatal DA release and axonal calcium signals.
4/n
🕒 Desensitisation of nAChRs is too slow (~100 ms) to explain ChI-dependent depression, which has an onset of <10 ms.
3/n
📉 imaging GCaMP6f or ASAP3 voltage sensor on DA axons, we found after nAChR activation, later calcium entry and depolarisation is limited.
2/n
🔬 Using a dual optogenetic approach, even minimal ChI activation (with blue light) - not enough to drive DA release - depressed DA release triggered by orange light!
1/n
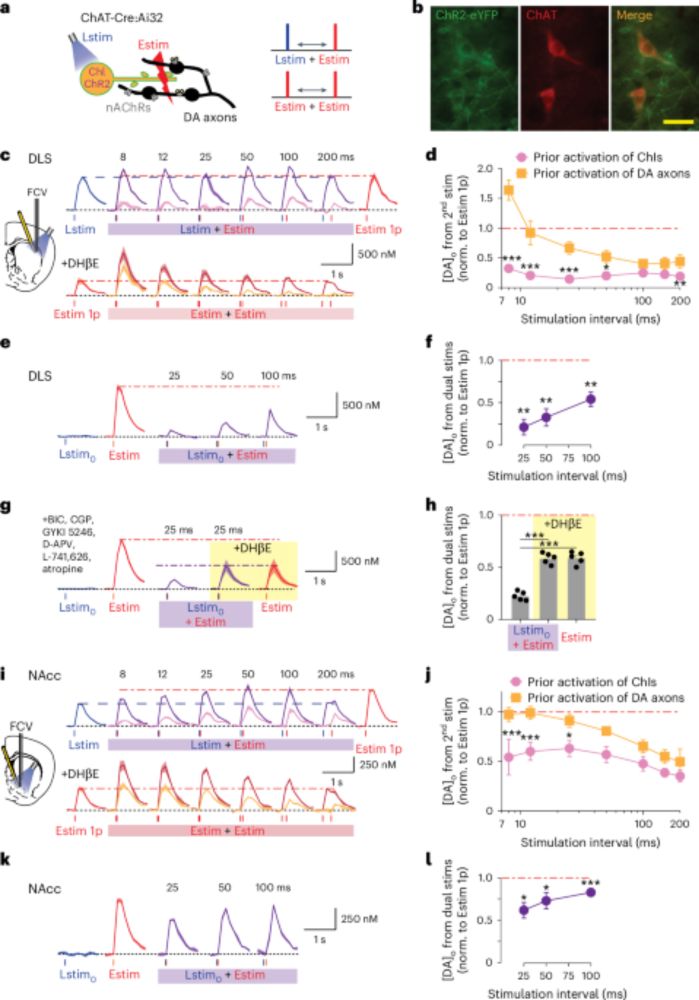
💡 We stimulated ChIs optogenetically, then applied electrical stimulation - DA release was depressed for up to 200 ms. The same depression occurred when ChIs and DA axons were co-activated electrically but only DA axons were stimulated optogenetically afterward.
🚨 Excited to share our new study in @natureneuro.bsky.social! We (+ @stephcragg.bsky.social) show that ChIs (via nAChRs) continuously, and dynamically DEPRESS dopamine release! @oxforddpag.bsky.social @hls.exeter.ac.uk www.nature.com/articles/s41...
17.03.2025 10:41 — 👍 32 🔁 11 💬 1 📌 3
8/n
🔥 Since nAChR activation prevents subsequent DA axon depolarisation, we need to rethink DA function models based on somatic firing - not all axons will respond to action potentials recorded at the cell body.
7/n
🔑 Conclusion: depression of DA release is the dominant effect at lower nAChR activation levels, although ChIs may be able to trigger DA release in some cases (enough active nAChRs, high synchrony of ChIs actions).
6/n
🧠 A computational model predicted:
1️⃣ Fluctuating ChI activity → inverse DA deviation
2️⃣ Multiphasic ChI activity → depression of phasic DA release during learning
5/n
🐭 In vivo, inhibiting nAChRs enhanced striatal DA release and axonal calcium signals.
4/n
🕒 Desensitisation of nAChRs is too slow (~100 ms) to explain ChI-dependent depression, which has an onset of <10 ms.
3/n
📉 imaging GCaMP6f or ASAP3 voltage sensor on DA axons, we found after nAChR activation, later calcium entry and depolarisation is limited.
2/n
🔬 Using a dual optogenetic approach, even minimal ChI activation (with blue light) - not enough to drive DA release - depressed DA release triggered by orange light!
1/n
💡 We stimulated ChIs optogenetically, then applied electrical stimulation - DA release was depressed for up to 200 ms. The same depression occurred when ChIs and DA axons were co-activated electrically but only DA axons were stimulated optogenetically afterward.