How is it that a paper that claims to show how to do target trial emulation does not address confounding by indication and its ramifications for data collection? www.jclinepi.com/article/S089... #EpiSky #StatsSky
28.02.2026 16:17 — 👍 21 🔁 6 💬 4 📌 1How is it that a paper that claims to show how to do target trial emulation does not address confounding by indication and its ramifications for data collection? www.jclinepi.com/article/S089... #EpiSky #StatsSky
28.02.2026 16:17 — 👍 21 🔁 6 💬 4 📌 1Practical elements to consider when emulating a target trial - Journal of Clinical Epidemiology www.jclinepi.com/article/S089...
28.02.2026 16:04 — 👍 4 🔁 2 💬 0 📌 0Brilliantly written with one small caveat: Towards the end the article implies that observational data may be useful for studying heterogeneity in treatment effects. Don't know why that would be the case. But great work Darren! #StatsSky #EpiSky
26.02.2026 13:48 — 👍 13 🔁 2 💬 1 📌 0We should rename these types of funnel plots to ‘avalanche plots’
24.02.2026 07:35 — 👍 25 🔁 4 💬 0 📌 0My own university has a "strategic goal" to increase research "outputs" by 10% annually (7 year doubling time). Your university is almost surely working towards the same. University rankings are now the main driver of this damaging institutional behavior. Publication is an out-of-control arms race.
16.07.2025 07:34 — 👍 144 🔁 39 💬 18 📌 9Too many meta-analyses have findings equivalent to: “If you average the cost of a loaf of bread, car insurance for a year and a movie ticket, you get $752.36”
26.01.2026 11:41 — 👍 52 🔁 11 💬 2 📌 1We are excited to share a recently published article in Clinical Trials which investigated confirmatory evidence supporting single pivotal trials for new drug approvals. (1/8)
03.11.2025 18:35 — 👍 0 🔁 1 💬 1 📌 0
Assessing outcomes emerging after conversion to regular approval for cancer drug indications granted accelerated approval, 1992-2021 url: academic.oup.com/jnci/article...
30.10.2025 16:10 — 👍 0 🔁 0 💬 0 📌 0
La dernière étude d'OpenAI prouve mathématiquement que les grands modèles linguistiques produiront toujours des résultats plausibles mais faux (hallucinations), même avec des données parfaites, à cause de la nature statistiques des algorithmes en jeu.
www.computerworld.com/article/4059...

Someone was trying to take a portrait of their two fave Chickens, and this happened.
20.09.2025 06:59 — 👍 5507 🔁 1574 💬 83 📌 216
CRISPR is amazing
21.09.2025 12:56 — 👍 108 🔁 14 💬 3 📌 4
C'est joli les scores, ça fait des publications, ca donne l'air malin, ca dit que les patients tres malades sont très malades et ont plus de risque de mourir, c'est poussé par des KOL
Et quand on les évalue correctement...

www.medrxiv.org/content/10.1...
INSPECT-SR: A tool for assessing trustworthiness of randomised controlled trials.

Alzheimer : polémique autour du Leqembi, un médicament à quelques milliards d’euros
Par @olivierhertel.bsky.social
If you are analyzing clinical data, you almost certainty don't need a multivariable model unless you are building a prognostic model or asking causal questions. Statistics shouldn't be a performance.
04.07.2025 13:43 — 👍 41 🔁 6 💬 5 📌 2
People often say their goal is to identify “risk factors”. But what does that mean? Some people use the term to indicate potential causes of outcomes. Then just say cause. Others use it to identify predictors of outcomes. Then just say predict. And, sadly, too many others use it as shorthand for factors that are “statistically associated” with outcomes. In this case, say nothing at all, since this “goal” has no clinical utility whatsoever (beyond what it might suggest about causation or prediction). So once you have framed your research question as description, prediction, causation or measurement, there is no longer a need to talk about risk factors. It's basically just a catch-all phrase to cover up muddy thinking.
Relevant:
(from statsepi.substack.com/p/sorry-what... ICYMI)
I'm not saying you can't generate a hypothesis from data.
I'm just saying that generating a hypothesis from the entirety of human knowledge that preceded your data is a much safer bet.
Read our paper in JAMA Internal Med with a great editorial. Wonderful collaboration with @gcabanac.cpesr.fr
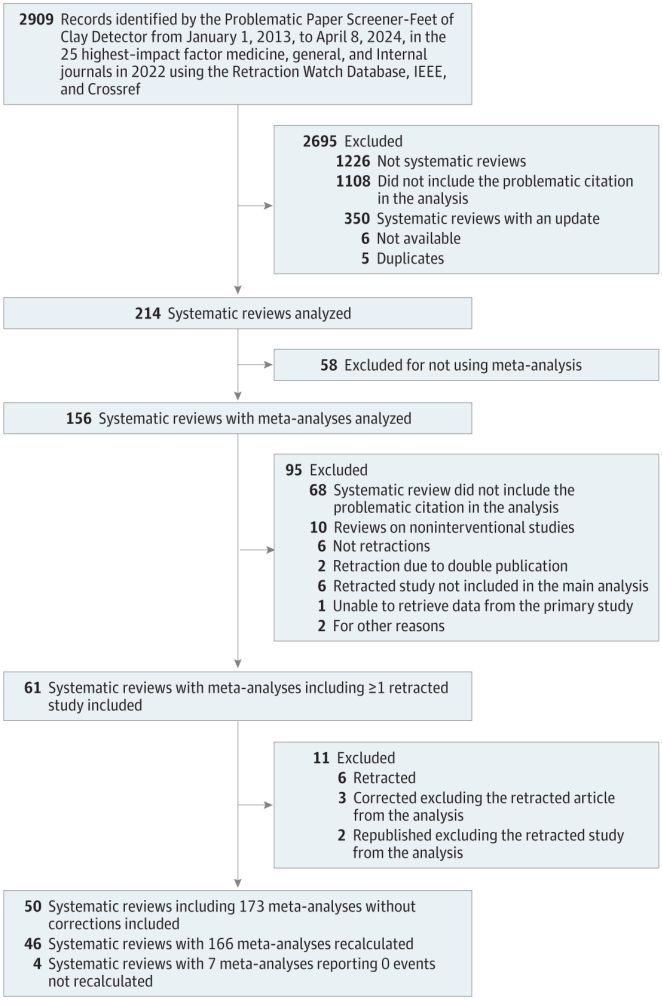
Retracted papers are included in meta-analyses published in HIF journal. Inclusion of the retracted studies changed
effect estimates.
jamanetwork.com/journals/jam...

Triangulation of evidence in medical research explained, in work led by Sirena Gutierrez and @mariaglymour.bsky.social Will do a thread when I get a moment
link.springer.com/article/10.1...
Provenance and funding of extremely cited biomedical papers published in 2003- 2004, 2013-2014 and 2023-2024
09.03.2025 07:10 — 👍 5 🔁 3 💬 0 📌 0Definitions of Validity Terms for Use in Discussions of Randomized Controlled Trials - Journal of Clinical Epidemiology www.jclinepi.com/article/S089...
09.03.2025 20:27 — 👍 10 🔁 2 💬 0 📌 0
Analysis of indications for selectively missing results in comparative registry-based studies in medicine: a meta-research study
08.03.2025 06:03 — 👍 1 🔁 1 💬 1 📌 0
Image shows website.
Viewpoint: Proposals to lower or eliminate the @fda.gov's preapproval effectiveness requirements for drugs are unnecessary and dangerous, as they undermine patient safety and public health. https://ja.ma/4brnk39
06.03.2025 16:35 — 👍 15 🔁 7 💬 0 📌 1
ICYMI: ‘Arbitrary’ Hodgepodge of Composite Endpoints Used Across ASCVD Trials
23.02.2025 21:40 — 👍 0 🔁 2 💬 0 📌 0This is sad...
22.02.2025 09:33 — 👍 25 🔁 6 💬 2 📌 0
doi.org/10.1002/sim....
Our paper "A Comparison of Statistical Methods for Time-To-Event Analyses in Randomized Controlled Trials Under Non-Proportional Hazards" got published today 🎉
We describe commonly used methods, and compare their performance in a simulation study across different scenarios.

Figure. Regression Discontinuities of Nirmatrelvir-Ritonavir Prescription, COVID-19–Related Hospitalization, and All-Cause Hospitalization and Death Rates per 100 000 Older Adults, April 1–November 30, 2022, Ontario
A more than doubled rate of nirmatrelvir-ritonavir prescriptions was not associated with reductions in hospitalizations or mortality among highly vaccinated older adults in Canada.
ja.ma/3ENOh4U
#MedSky

"We cannot think that our AI systems will not be biased. There will always be a risk of bias."
María Villalobos-Quesada, a researcher at Leiden University Medical Centre, discusses the need for transparent and consistent reporting of quality for #AI tools with JAMA+ AI.
#MedSky
Author’s reply : “The importance of properly specifying your target trial emulation: commentary on Mésidor et al.” - Journal of Clinical Epidemiology www.jclinepi.com/article/S089...
16.02.2025 19:13 — 👍 1 🔁 1 💬 0 📌 0