
Congratulations Dr. Lauren Ehehalt on a fantastic thesis defense! We're so excited to see all your future work at Eastman Chemical Co.! 🥳🎉🎓
21.05.2025 16:15 — 👍 5 🔁 1 💬 0 📌 0
Congratulations Dr. Lauren Ehehalt on a fantastic thesis defense! We're so excited to see all your future work at Eastman Chemical Co.! 🥳🎉🎓
21.05.2025 16:15 — 👍 5 🔁 1 💬 0 📌 0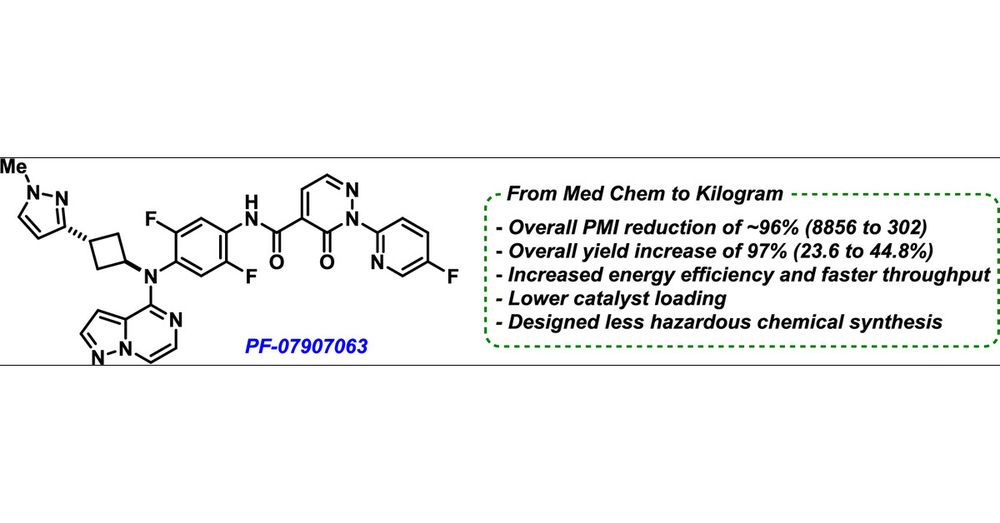
Another #OPRD asap to highlight is this one from #PfizerChemistry showing a kilo scale route to complex API with all kinds of cool - small hets (pyrazoles!) cyBu, Weix cross electrophile coupling, key synthesis improvements driven by reaction safety…so much to discover in this one #ChemSky
15.04.2025 15:06 — 👍 6 🔁 1 💬 0 📌 0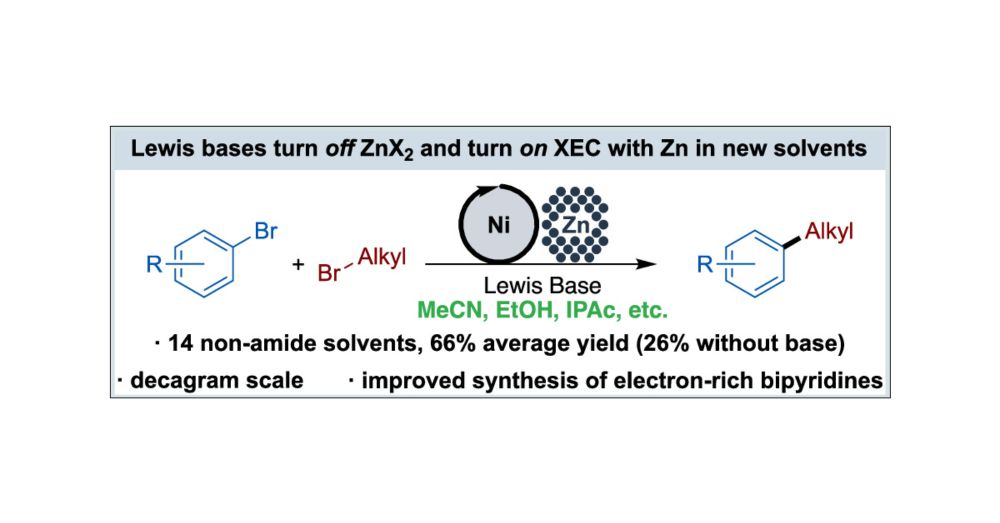
Our work on the translation of Nickel-Catalyzed C(sp2)–C(sp3) Cross-Electrophile Coupling to Non-Amide Solvents is now online in Org. Lett.! @pubs.acs.org Congrats to Brett, Julianna, Michelle, and wonderful collaborators at @novartis.bsky.social Check it out! doi.org/10.1021/acs.orglett.5c01011
15.04.2025 15:50 — 👍 12 🔁 2 💬 0 📌 0
Congrats to Julianna for receiving an Outstanding Chemistry Teaching Assistant Award for your work with Chem 636 and the NMR facilities! Well deserved! 🎉
14.03.2025 21:30 — 👍 4 🔁 1 💬 0 📌 0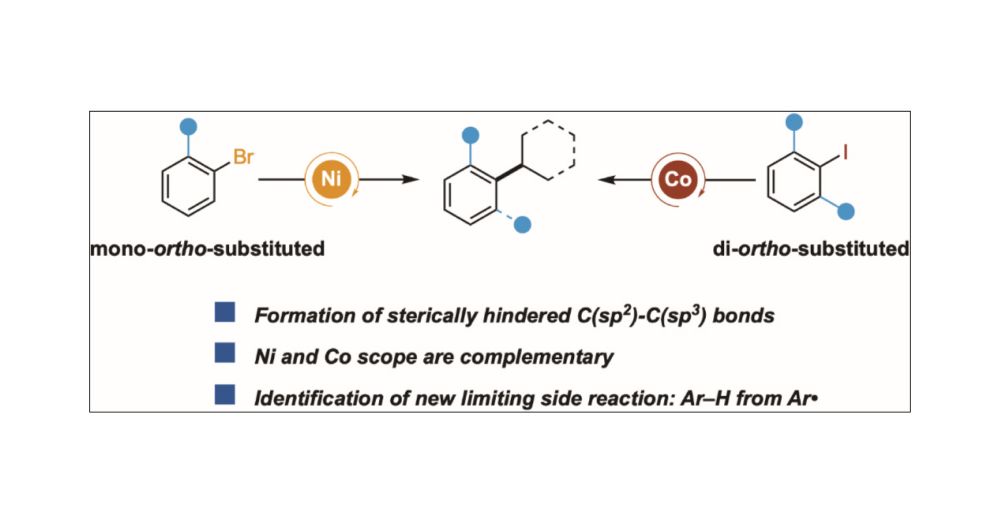
Our work on Ni- and Co-catalyzed Cross-Electrophile Coupling to Form Sterically Hindered C(sp2)–C(sp3) Bonds is now online at J.A.C.S.! @pubs.acs.org Congrats to Tianrui, Anthony, Kasturi, and our collaborators Madeline (@kozlowskigroup.bsky.social) and @novartis.bsky.social doi.org/10.1021/jacs...
07.03.2025 20:09 — 👍 12 🔁 3 💬 0 📌 1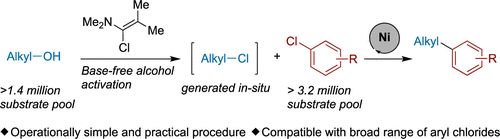
Our work on in-situ generation of alkyl chlorides using Ghosez’s reagent and coupling with aryl chlorides is now online at Org. Lett. Congrats Ben! @pubs.acs.org Check it out! doi.org/10.1021/acs.orglett.4c04676 #chemsky
24.01.2025 17:10 — 👍 5 🔁 0 💬 0 📌 0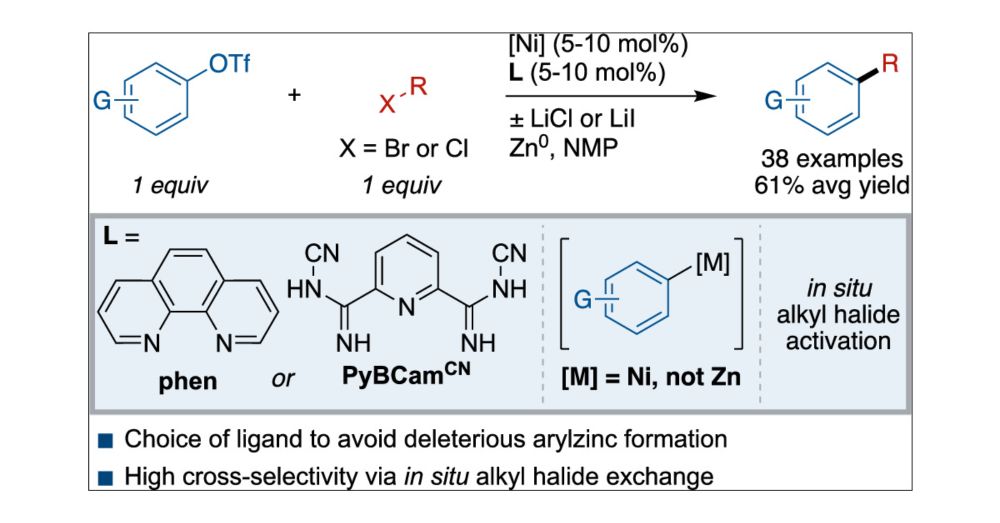
Our work on Ni-Catalyzed Cross-Electrophile Coupling of Aryl Triflates with Alkyl Halides is now online at JACS @pubs.acs.org. Congrats to Seoyoung, Matt, Ben, and Daniel! doi.org/10.1021/jacs.4c14769
13.01.2025 20:18 — 👍 23 🔁 3 💬 1 📌 0
Welcome to Abi! We're excited to see all your awesome work in the group! 🥳✌️
08.01.2025 18:35 — 👍 4 🔁 0 💬 0 📌 0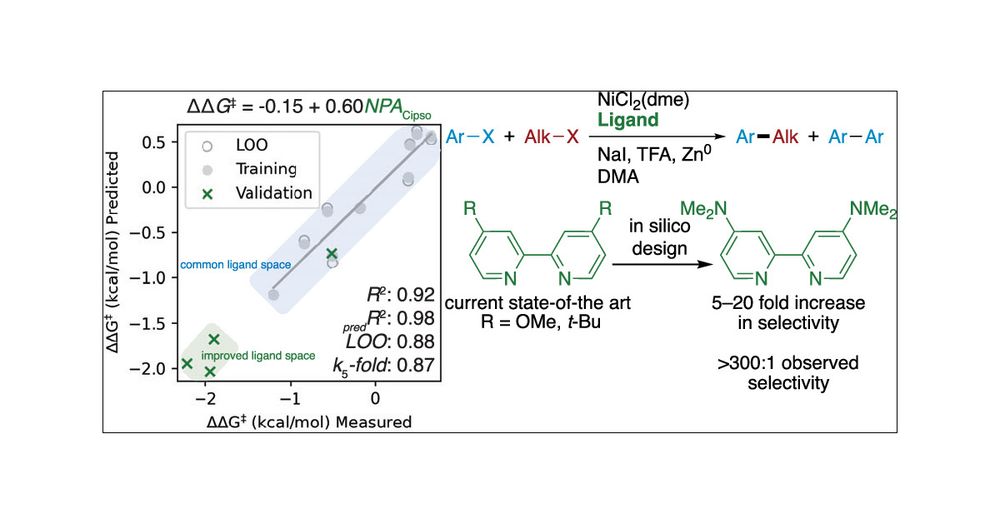
4a/ #12DaysofPapers #ChemSky. This 2024 favorite is a #PfizerChemistry collab with the Weix (Wisconsin) and Sigman (Utah) labs towards computationally designed ligands for Ni-catalyzed cross electrophile coupling. Improvements in selectivity up to 300% #ChemCollabs
11.12.2024 19:26 — 👍 3 🔁 4 💬 0 📌 0
We're incredibly proud to say that our review of Cross-Electrophile Coupling: Principles, Methods, and Applications in Synthesis is now online and open access at Chem Rev! Congrats to the team for this labor of love (and data!) pubs.acs.org/doi/10.1021/...
12.12.2024 22:18 — 👍 37 🔁 10 💬 0 📌 2