www.organic-chemistry.org/Highlights/2...
Douglass features Reduction: The Bisai Synthesis of Oridamycin A
21.09.2025 15:13 — 👍 0 🔁 1 💬 0 📌 0
In Co-catalyzed hydroformylation, where substantial phosphine rearrangement is required to stabilize the precatalyst and structures along the catalytic cycle, MOF-heterogenized phosphines reproduce homogeneous performance while offering recyclability and minimizing leaching (< 0.7 ppm Co).
06.07.2025 17:14 — 👍 0 🔁 0 💬 0 📌 0
We show via solid-state NMR studies that the phosphine ligands enjoy a high degree of mobility within the super-cages, which permits them to furnish mechanistically homogeneous and functionally heterogeneous transition-metal catalysts.
06.07.2025 17:13 — 👍 1 🔁 0 💬 0 📌 0
Thieme E-Journals - Synthesis / Abstract
Thieme E-Books & E-Journals
#newpaper Preferential 5-endo-trig and 5-exo trig cyclizations of silyl radicals with a MOF-based Rh(II) porphyrin catalyst enables chemoselective 1,3-diol formation for olefins that contain multiple olefins: www.thieme-connect.com/products/ejo...
Congrats to all authors (Gupta, Deng, Ding, Qiu)!
05.05.2025 22:11 — 👍 2 🔁 0 💬 0 📌 0
Our mechanistic proposal rationalizes why MOF-supported Rh(II) porphyrins are thermal hydrofunctionalization catalysts while molecular analogues prove unreactive. It also showcases the cooperation that can be achieved within a MOF matrix which precisely arranges Rh(II) centers in 3D space.
31.03.2025 17:51 — 👍 0 🔁 0 💬 0 📌 0
Via EPR and NMR spectroscopic analyses, kinetics, isotope labeling, IR spectroscopy, molecular model systems, radical trapping, digestion studies, quantum chemistry and molecular dynamics, we could get a rare level of insight into the mechanistic details of a heterogeneously catalyzed reaction.
31.03.2025 17:50 — 👍 2 🔁 0 💬 0 📌 0
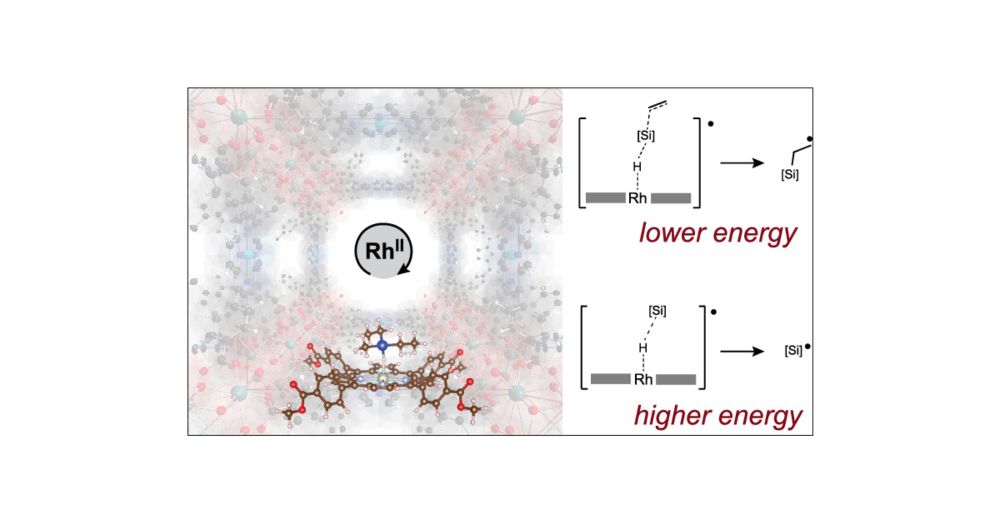
3-Center-3-Electron σ-Adduct Enables Silyl Radical Transfer below the Minimum Barrier for Silyl Radical Formation
Transition-metal-catalyzed cleavage of the Si–H bond in silanes to yield silyl radicals requires substantial amounts of energy, which are commonly supplied by photons. For Rh(II) porphyrins, efficient...
Our mechanism study of a MOF-catalyzed hydrosilylation reaction is now available in #JACS.
Congratulations to all authors!
Zihang Qiu, Paolo Cleto Bruzzese, Zikuan Wang, Hao Deng, Markus Leutzsch, Christophe Farès, Sonia Chabbra, PhD, Frank Neese, Alexander Schnegg
pubs.acs.org/doi/10.1021/...
31.03.2025 17:49 — 👍 9 🔁 2 💬 2 📌 0
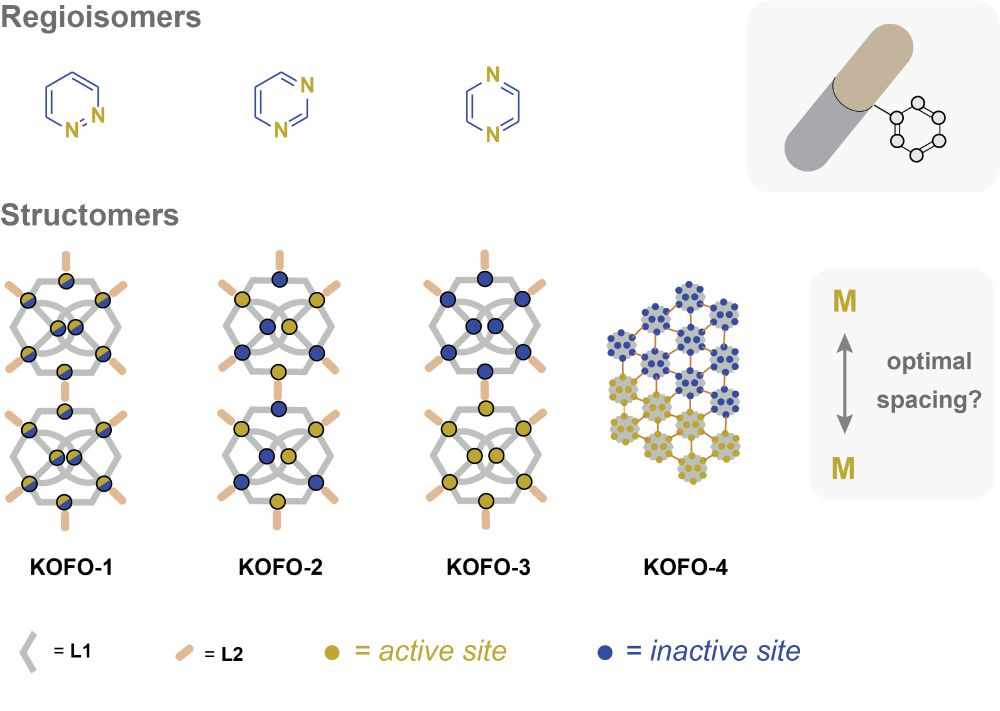
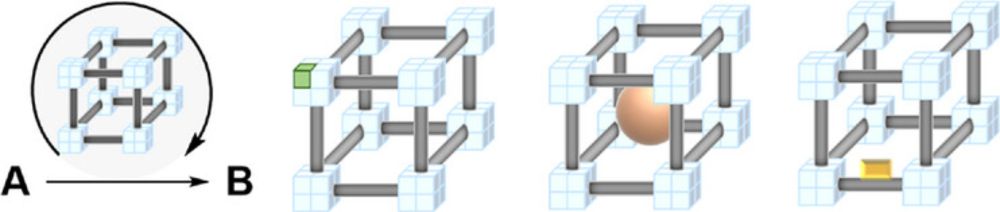
The graphic shows three regioisomers of diazines in the top row and shows a sketch of a pill with an attached arene. The latter alludes to the fact that the ability to prepare individual regioisomers permits researchers to optimize the position of nitrogen atoms in arene rings for applications in medicinal chemistry.
The bottom row shows simplistic representations of four different structomers of metal-organic frameworks. All four structomers have the same composition and are isostructural, but differ in how "active" and "inactive" metal sites are arranged within the structure. Selective preparation of individual structomers thus permits one to optimize metal arrangements in extended structures.
Our work on the development of structomers is now available as a preprint: chemrxiv.org/engage/chemr...
Congratulations to the authors Qiming Jin, Paolo Cleto Bruzzese, Alessandro Vetere, Claudia Weidenthaler, Eko Budiyanto, Mir Henglin, Nils Nöthling and Alexander Schnegg!
09.01.2025 09:55 — 👍 1 🔁 1 💬 0 📌 0
Photochemist, former Leopoldina Postdoc, Husband, Father of 2, Chemistry Professor @ JGU Mainz, board member of the GDCh photochem. section https://www.ak-kerzig.chemie.uni-mainz.de/
Associate Professor, Department of Chemistry, University of Warwick
Framework materials, total scattering & Fourier transform enthusiast │ Curator of patterns and alternative crystallographic teaching at behance.net/specialdefects
https://orcid.org/0000-0002-6817-0810
Associate Professor at York University | 🇨🇦 organic chemist, mom 👶🏻🐶, foodie, swiftie | she/her
Assistant Professor at MGH. PI if the Brugarolas lab. Radiochemist, PET tracer developer, husband, dad.
https://brugarolas.mgh.harvard.edu/
Lecturer @University of Manchester. Interested in low-valent transition metal complexes, organoaluminium chemistry and cyclopropanes
Professor of Chemistry @ INSA ROUEN Normandie - views are my own.
Associate Professor at Westlake University l Associate Editor of SmartMat @smartmat.bsky.social. Focus on Molecular-Strain Engineering / Supramolecular Organic Functional Assembly
liu.westlake.edu.cn
Professor of Theoretical Chemistry @sorbonne-universite.fr & Director @lct-umr7616.bsky.social| Co-Founder & CSO @qubit-pharma.bsky.social | (My Views)
https://piquemalresearch.com | https://tinker-hp.org
CNRS Researcher in France enjoying cycloadditions, catalysis, natural products, new technologies for chemistry and data science. Wine and food enthusiast!
bsm.unistra.fr
Asst. Prof. of Chemistry @ UMass Boston • Inorganic / Organometallic Chemist • Ain't no bonding like Metal-Metal Bonding
A young research group at York University in Toronto. Specializing in synthesis and catalytic activation of fluorinated molecules.
http://leresearchgroup.com
The research interests of the Breher group centre around the synthesis, structure, bonding and reactivity of organometallic and coordination compounds.
https://www.aoc.kit.edu/breher/
https://www.kit.edu/
Lecturer in Inorganic Chemistry at UoM | Organometallic/main group chemist | First generation academic | www.abbenseth-lab.com
We are Phosphorus Chemsitry enthusiasts.
From Ligand Design with Phosphaalkenes to
Phosphinidene Transfer and Phosphaalumenes.
Located at the LIKAT Rostock.
Chair of the COST Action CA21101: Research network in Europe dedicated to COnfined Molecular SYstems (COSY). Permanent Researcher at IFF-CSIC.
https://cost-cosy.eu
https://www.cost.eu/CA21101
@cosy-action.bsky.social
Research group leader at ETH Zurich passionate about organic chemistry and science in general. Outside work I enjoy parenting, listening to music (percussionist) and travelling to sunny places with good food.
Assistant Professor of Chemistry at DGIST 🇰🇷 | Main Group Chemist | formerly: Cornella group at MPIKOFO (postdoc), Radosevich group at MIT (PhD)
http://www.hmoonlab.com
Inorganic chemistry and photocatalysis @bathchem.bsky.social
https://scottchemistry.wixsite.com/scott-research-group
Assoc. Professor @JohnsHopkins | PI of Kempa Group | Co-Director of HIQT | Interests: materials, inorganic, & physical chemistry | From 🇵🇱 to 🇺🇸