Thanks to @lisavondung.bsky.social, Axel Jacobi von Wangelin, Peter Burger, Dieter Schaarschmidt and helpers for putting together such an amazing event.
27.09.2025 16:10 — 👍 2 🔁 0 💬 0 📌 0


Soti and Sina presented their latest results at the NDDK this week. After just two months in the lab, Sina secured her first conference prize! Huge congratulations to her 🥳! We had a wonderful time, topped off by a buffet and drink selection of unparalleled quality and abundance.
27.09.2025 16:08 — 👍 10 🔁 1 💬 2 📌 0
Good luck Jasmin =)
25.09.2025 08:07 — 👍 2 🔁 0 💬 0 📌 0
Personal | Dezernate | Verwaltung | TU Chemnitz
Dezernat Personal: Personal
We are looking for a highly motivated PhD student to join our ERC project AGILE at the interface of inorganic, organic and organometallic chemistry. A vibrant Team, cool chemistry and everything takes place in the cultural capitol of Europe CHEMnitz. Apply now: www.tu-chemnitz.de/verwaltung/p...
22.09.2025 12:32 — 👍 7 🔁 10 💬 0 📌 0

📢pls share
We are hiring! New opening for a W2 Professor in "experimental inorganic chemistry" @unibonn.bsky.social
Deadline Oct. 10 t.co/EqMLA0fCuC
19.08.2025 20:09 — 👍 33 🔁 24 💬 3 📌 3
Beautiful work! Congratulations Crispin and team.
14.08.2025 08:58 — 👍 1 🔁 0 💬 0 📌 0
Highly recommended!
14.08.2025 08:45 — 👍 0 🔁 0 💬 0 📌 0
Awesome work =)
06.08.2025 09:23 — 👍 0 🔁 0 💬 0 📌 0
So good to see this amazing work in print, congratulations =)
01.08.2025 13:21 — 👍 1 🔁 0 💬 0 📌 0
https://pubs.acs.org/doi/full/10.1021/acs.inorgchem.5c02152
Congrats André and all the authors involved in this new generation of SBDIPY and BIDIPY and their application in photocatalysis.
t.co/0B4FRFBAUy
31.07.2025 13:30 — 👍 14 🔁 2 💬 1 📌 0
Very nice Peter, congrats
27.07.2025 12:13 — 👍 1 🔁 0 💬 0 📌 0
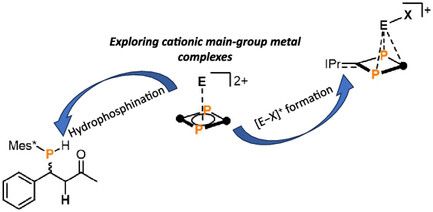
π‐Complexes of Main‐Group Metal Cations: Exploration of Lewis Acid Reactivity
A series of cationic main-group complexes was obtained using the neutral, zwitterionic ligand IDP. The Lewis acidity of these complexes is explored by catalytic hydrophosphinations and halide additio...
Our latest publication reports the successful isolation of π-stabilized tetryliumylidenes [E–X]+ starting from pyramidanes based on a biradicaloid ligand . This milestone was made possible thanks to David’s relentless efforts . Proud of the team!
26.07.2025 13:35 — 👍 15 🔁 2 💬 1 📌 0
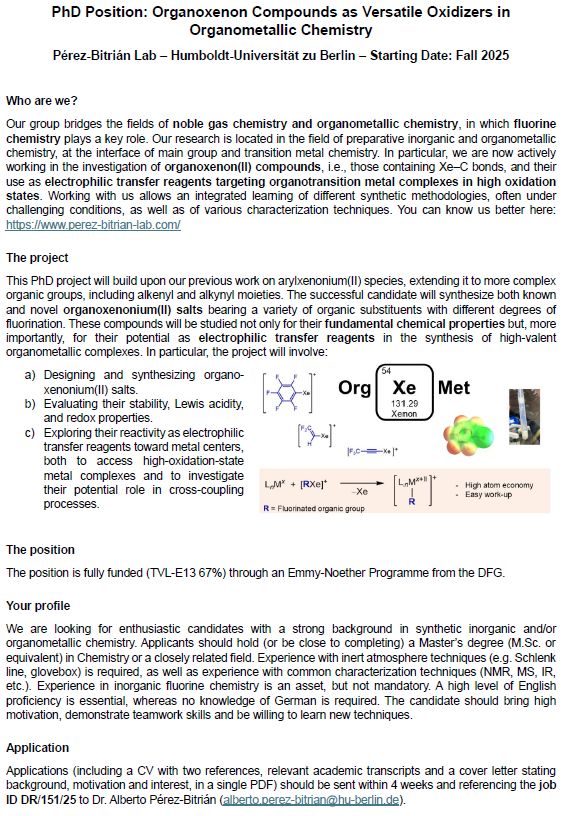
PhD POSITION OPEN! We are looking for someone enthusiastic to join our team at @humboldtuni.bsky.social! If you want to work in the frontier between noble gas chemistry and organometallic fluorine chemistry, this is for you! #TeamXenon
Info here: haushalt-und-personal.hu-berlin.de/de/personal/...
23.07.2025 10:05 — 👍 4 🔁 5 💬 0 📌 1
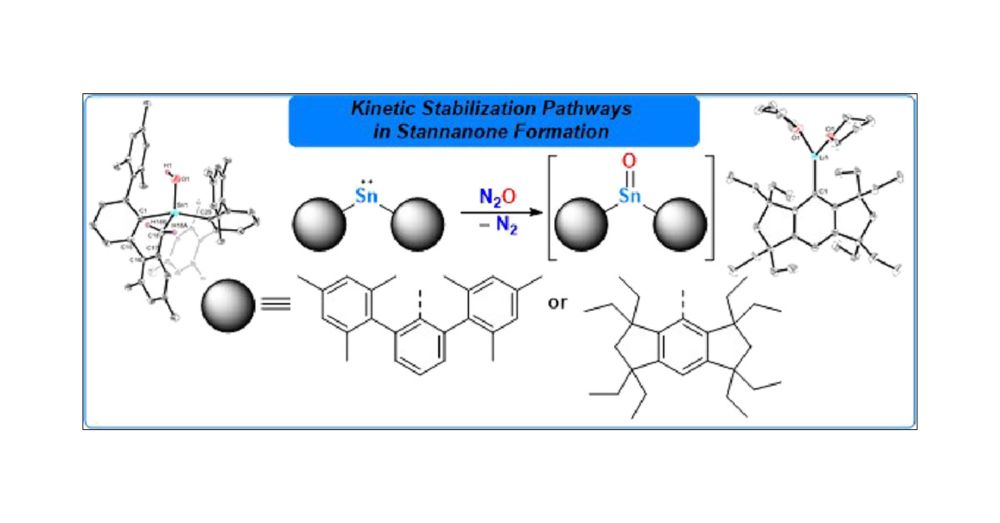
Kinetic Stabilization in Diaryl-Substituted Stannylenes: N2O Reactivity, Intramolecular C–H Activation, and Crystalline (Eind)Li(THF)2 as a Versatile Precursor in Tin Chemistry
The reactivity of the kinetically stabilized stannylene (MesTer)2Sn (1) (MesTer = –C6H3-2,6-(2,4,6-Me3-C6H2)2) toward N2O is revisited, yielding the terminal tin(IV) hydroxide 2 via formal intramolecular C(sp3)–H activation of a putative terminal stannanone intermediate. By switching to Eind ligation (Eind = 1,1,3,3,5,5,7,7-octaethyl-s-hydrindacen-4-yl) at the tin center, the synthesis and characterization of the crystalline lithium salt (Eind)Li(THF)2 (3) is reported, serving as a straightforward precursor for the clean generation of the corresponding stannylene (Eind)2Sn (4). Compound 4 can be further cleanly converted into the heteroleptic Eind/halide stannylene (Eind)SnCl (6). Both 4 and 6 serve as suitable precursors for the synthesis of the heteroleptic s-hydrindacene-/amido-substituted stannylene (Eind)Sn{N(SiMe3)2} (5).
Excited to share our contribution to the special issue "Organometallic Chemistry Beyond the Transition Metals: Fundamentals and Applications of the P-Block" – now published @Organometallics! Check it out 👉 @pubs.acs.org #ChemSky
pubs.acs.org/doi/10.1021/...
22.07.2025 16:43 — 👍 36 🔁 7 💬 1 📌 0
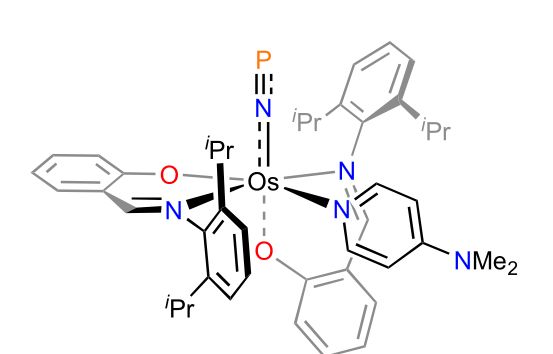
🎉 Just out in Nature Comm @natcomms.nature.com
"Unleashing phosphorus mononitride"
👉 www.nature.com/articles/s41...
Spearheaded by the Reinholdt group (Lund), we find that the elusive P≡N molecule finds new life in an Os complex and unlocks unique [NPS₂]²⁻, [NPCl]⁻, and aromatic [PN₄]⁻ species.
01.07.2025 12:29 — 👍 27 🔁 7 💬 0 📌 0
Wow, this is great! Congratulations
02.07.2025 20:14 — 👍 1 🔁 0 💬 1 📌 0
Congratulations Marcus, well deserved!
01.07.2025 05:29 — 👍 1 🔁 0 💬 1 📌 0
Awesome work David, Nicholas and team, congratulations!
26.06.2025 07:05 — 👍 3 🔁 0 💬 1 📌 0

Studienorganisation
Chiffre CHEM-PHARM-15179
We are hiring a new PhD student. If you love working with f-elements and early transition metals, plus living in a beautiful mountain area, please apply.
lfuonline.uibk.ac.at/public/karri...
23.06.2025 09:50 — 👍 9 🔁 5 💬 0 📌 0
Thank you so much Jose!
22.06.2025 10:21 — 👍 0 🔁 0 💬 0 📌 0
Thanks a lot David!
22.06.2025 10:20 — 👍 1 🔁 0 💬 0 📌 0
Thanks Gunnar, see you soon!
22.06.2025 10:19 — 👍 0 🔁 0 💬 0 📌 0
Chefkümmerer bei Carbolution
Inorganic Chemistry, Organometallic Chemistry, Nitrogen Activation. Werder Bremen Fan.
Ph.D @AK Meyer
🌍 Official account of the International Conference on Coordination Chemistry (ICCC). Updates for #ICCC46 (Odense, June 28–July 3, 2026).
Visit: iccc2026.com
A proud Manchester institution with teaching, research and social responsibility at the heart of everything we do.
research group @Max-Planck-Insitut für Kohlenforschung heterogeneous catalysis - MOF chemistry - metal phosphides
Inorganic chemistry group focussing on sustainability at University Tübingen
Leader of the group Receptor Biochemistry @ipbhalle.bsky.social
Plant-pathogen, receptors, #proteases, fungi, effectors, crop protection
Mom
🇨🇴🇩🇪
https://www.ipb-halle.de/en/research/independent-junior-research-groups/research-groups/receptor-biochemistry
Feodor Lynen Fellow @AvHStiftung
|| Postdoc @JonesResearch @Monash_Science
|| Postdoc @GHierlmeier
|| PhD @LabBraunschweig @Uni_WUE
Teaching-focused senior lecturer in chemistry at the University of Manchester, training the next generation of lab monkeys and arrow-pushers. Occasional dabbler in ChemEd research.
Inorganic chemistry and photocatalysis @bathchem.bsky.social
https://scottchemistry.wixsite.com/scott-research-group
Postdoctoral researcher @goicoecheagroup.bsky.social. Working on some cool main-group chemistry!! #TheParamagneticGuy
Ph.D. (2025) from @camposgroup.bsky.social
B. Sc. and M. Sc. in Chemistry from @humboldtuni.bsky.social
🧪 Senior Lecturer | U. of Strathclyde
📕 Author | “You Are Not a Fraud”
🎤 Host | The reid_indeed Podcast
🎥 CSO | Kineticolor Ltd
https://www.dr-marc-reid.com
News and updates from the Dielmann group - synthetic inorganic chemistry research lab. Account is managed by graduate students.
For further information also visit our homepage https://www.uibk.ac.at/en/aatc/ag-dielmann/
Organic Chemistry Lab at ISIC EPFL, NCCR Catalysis, SNE ChemBio
https://www.epfl.ch/labs/lcso/ https://www.linkedin.com/in/lcso-lab/
Starter packs - OrgChem https://go.bsky.app/NSEFPFJ https://go.bsky.app/GwH8t
- Chem. in CH https://go.bsky.app/CdvVFKj