I've heard this in multiple fields and can't say it is consistent with anyone I know who recently completed a PhD's experience
07.06.2025 19:38 — 👍 0 🔁 0 💬 0 📌 0
Congrats!
02.05.2025 01:30 — 👍 1 🔁 0 💬 0 📌 0
The color is impeccable
24.04.2025 03:57 — 👍 1 🔁 0 💬 0 📌 0
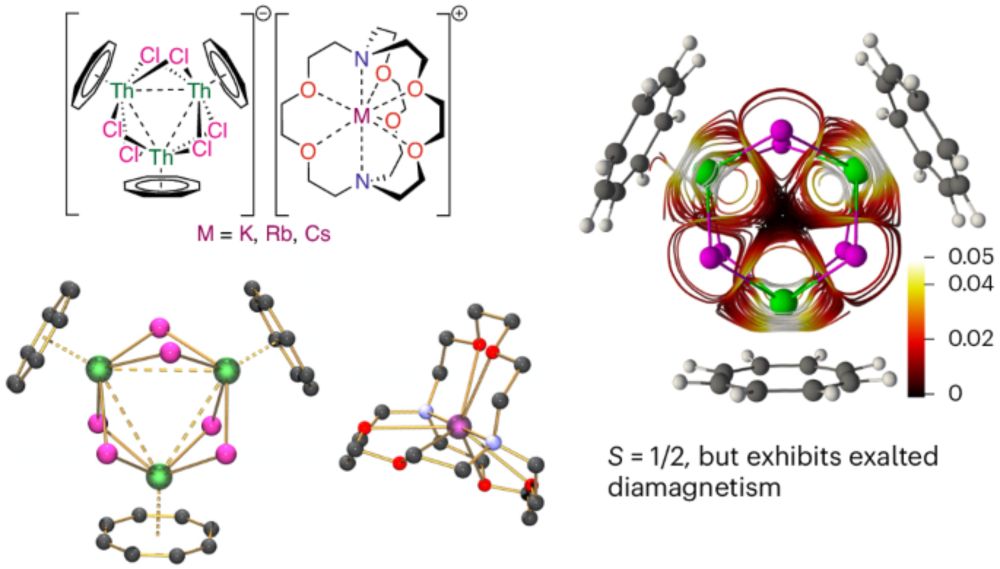
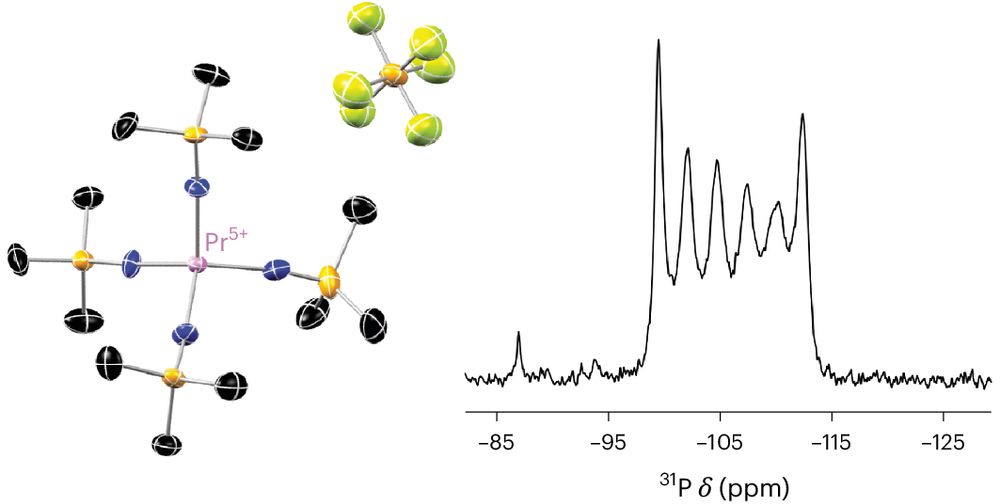
From @acboggiano.bsky.social, @f-orbitals.bsky.social, and co-workers, the first crystal structures of Pr(v) complexes.
The 5+ state was achieved through oxidation of the tetravalent imidophosphorane complex.
🔗CSD Entry TUNXOT: ccdc-info.com/4je9YdB
#FeaturedStructureFriday
@natchem.nature.com
18.04.2025 13:37 — 👍 11 🔁 2 💬 0 📌 0
Very nice!!
17.04.2025 12:47 — 👍 1 🔁 0 💬 0 📌 0

Mark your calendars for a thought provoking Solid-State Periodic TableTalks (Wednesday 4/16 at 12 pm PST/ 3pm EST) featuring Maxx Arguilla (@maxxsolidchem.bsky.social, UCIrvine) and Susan Kauzlarich (UCDavis). Register here:
unr.zoom.us/meeting/regi... @acs.org
11.04.2025 22:19 — 👍 5 🔁 3 💬 0 📌 0
Congrats! Great week for the f block in Nat Chem 😀
11.04.2025 12:45 — 👍 1 🔁 0 💬 1 📌 0
It can be found in the ACIE paper on Pr4, and also FigS67 in Nat Chem
08.04.2025 01:03 — 👍 1 🔁 0 💬 1 📌 0
Parliamentarian said no :/
02.04.2025 16:53 — 👍 2 🔁 0 💬 0 📌 0

Synthesis of triple-decker sandwich compounds featuring a M–M bond through cyclo-Bi5 and cyclo-Sb5 rings - Nature Chemistry
Synthesis of triple-decker sandwich compounds featuring a M–M bond through cyclo-Bi₅ and cyclo-Sb₅ rings, from Zhong-Ming Sun, Gernot Frenking, Lili Zhao, and their team:
https://bit.ly/4c7UqFt
#chemsky
24.03.2025 17:53 — 👍 13 🔁 1 💬 0 📌 0

This article Collection from Inorganic Chemistry, JACS and Organometallics honors the recently retired Distinguished Professor Philip Power of the University of California Davis.
Learn more: buff.ly/3XnrNxI
06.03.2025 16:00 — 👍 9 🔁 1 💬 0 📌 0

Interested to join the M3 team as a postdoctoral researcher on molecule-based magnets? Conducting metal-organic magnets? Switchable complexes? Porous MOF? We have a few positions available. @crpp-bordeaux.bsky.social
@univbordeaux.bsky.social aux.bsky.social. Contact us ASAP, @cleracr.bsky.social
01.03.2025 17:22 — 👍 4 🔁 2 💬 0 📌 0
🗑️
11.02.2025 03:06 — 👍 0 🔁 0 💬 0 📌 0

Out of Africa: celebrating 100 years of human-origins research
A landmark study reporting the discovery of Australopithecus africanus one century ago put the African continent at the centre of the story of humanity.
A 100 years ago, Nature published a landmark study reporting the discovery of Australopithecus africanus; it put the African continent at the centre of the story of humanity
In our editorial this week, we look back to 1925
🧪
@nature.com @natureportfolio.nature.com
www.nature.com/articles/d41...
05.02.2025 22:42 — 👍 31 🔁 11 💬 2 📌 0
I have wondered why current admin is not interested in bolstering scientific funding given how much they seem to care about staying competitive globally... Seems like it would be a pretty convincing argument
05.02.2025 05:23 — 👍 1 🔁 0 💬 0 📌 0
😂 I've written this footnote before, specifically for reviewer #2
31.01.2025 16:06 — 👍 1 🔁 0 💬 0 📌 0

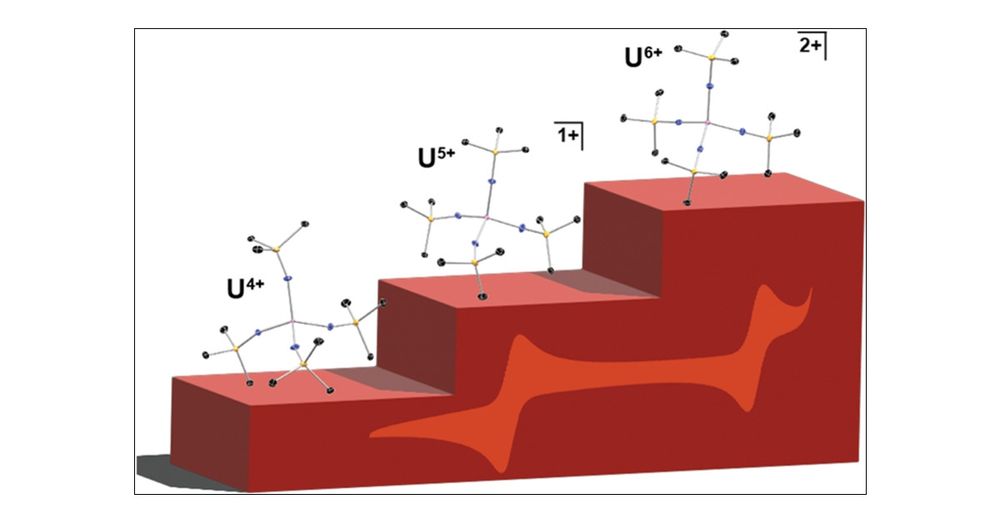
U4+/5+/6+ in a Conserved Pseudotetrahedral Imidophosphorane Coordination Sphere | Inorganic Chemistry pubs.acs.org/doi/10.1021/... La Pierre and co-workers @InorgChem #uranium #456cationic #imidophosphorane #pseudotetrahedral
29.01.2025 08:15 — 👍 6 🔁 2 💬 0 📌 0
For me it's usually when all the figures and tables are there and actually have captions
16.01.2025 20:26 — 👍 0 🔁 0 💬 0 📌 0
A fantastic team!
15.01.2025 20:27 — 👍 1 🔁 0 💬 0 📌 0

Redox and Photochemical Reactivity of Cerium(IV) Carbonate and Carboxylate Complexes Supported by a Tripodal Oxygen Ligand | Inorganic Chemistry pubs.acs.org/doi/10.1021/... Williams, Leung, and co-workers @InorgChem #cerium #carbonate #carboxylate #tripodal
15.01.2025 14:33 — 👍 0 🔁 1 💬 0 📌 0
@natureportfolio.bsky.social
15.01.2025 02:19 — 👍 0 🔁 0 💬 0 📌 0
The pay wall 😂 impeccable
15.01.2025 02:15 — 👍 0 🔁 0 💬 1 📌 0
Excellent news, congrats Cory!!
14.01.2025 20:46 — 👍 1 🔁 0 💬 0 📌 0


Snow in Atlanta 🥶
10.01.2025 20:40 — 👍 1 🔁 0 💬 0 📌 0
Organometallic Chemist | Postdoc at FAU Erlangen-Nürnberg
Inorganic chem researcher @stieberlab | CPP alum | LSAMP NSF Scholar | Queer In Stem | He/Him/His
Synthetic Inorganic and Materials Research Team @ UofR
Est. 2021
https://sas.rochester.edu/chm/groups/barnett/
Deputy NMR Facility Manager (Molecular Sciences Research Hub, Imperial College London) 🏳️🌈
Science writer and senior correspondent at C&EN. Opinions here are mine and mine alone (she/her).
Signal: bethanyhalford.29
Organometallic chemistry at University of Chinese Academy of sciences
Research, news, and information from the Journal of the American Chemical Society: pubs.acs.org/JACS
Old process chemist - I make drugs by the metric fuckton
Ready to make the free pharmaceuticals that save lives in the glorious corporation free future
Follow all scientists
Ask me about drugs or chemistry
Flagship journal for @rsc.org, open access with no publication fee, all topics in chemistry. chemicalscience-rsc@rsc.org
Celebrating our 15th year in 2025! 🌐 Website: rsc.li/chemscience
Organometallic Chemist
PostDoc at Yale with Nilay Hazari
PhD UC Irvine with Bill Evans
Research Fellow in the Liddle group at the University of Manchester. Actinide metal-metal bonding, actinide-pnictogen chemistry, and actinide magnetism ☢️☢️🧲
Scientific Editor, RSC. Views my own.
Background in computational chemistry and education.
Sci-fi, blackgaze, queer horror books, MMOs - including an insistent love of Phantasy Star Online 🏳️⚧️
Scientific Editor of @chemicalscience.rsc.org, the flagship journal of the Royal Society of Chemistry. Diamond open access! | bioinorganic chemist | PhD Stanford University, post-doc University of York | wife, mum, gardener, Christian | she/her
Grad Student in Comp Sci @ UIUC @uofigrainger.bsky.social | Former Sr. Research Asst. in Radiation Physics @ Univ. of Texas MD Anderson Cancer Center @mdanderson.bsky.social, developed AI for RPA https://rpa.mdanderson.org | 🏳️🌈 he/him | Views are my own
Postdoc at Uni Iowa. Computational Chemist.
Grad student in La Pierre Group at Georgia Tech. Actinides, cats, and crystallography lover.
Bird Enthusiast. Runner. Gamer. PhD candidate in #compchem at UIOWA.
Arnold O. Beckman Postdoctoral Fellow at Johns Hopkins University | Klausen Research Lab |Ph.D. from University of Virginia, Gilliard Lab Alum | Main-Group Synthetic Chemistry | Polymers
Nature Portfolio’s high-quality products and services across the life, physical, chemical and applied sciences is dedicated to serving the scientific community.
Prof @USask. Research includes nanochemistry, catalysis, and staring at X-rays. Dad of two teenagers and 1 cat, avid reader, and movie buff. Social introvert and lover of bad dad jokes.