Huge congratulations Darren and team!
20.02.2026 08:53 — 👍 0 🔁 0 💬 1 📌 0
Congrats Meera and co. This is ace - and beautiful EPR spectra too!
20.02.2026 08:52 — 👍 1 🔁 0 💬 0 📌 0
Very cool work! Congrats
20.02.2026 08:50 — 👍 2 🔁 0 💬 1 📌 0
Matt, Imogen and Mickey’s paper detailing the synthesis and reactivity of the first neutral Al(I) trimer is out now in @natcomms.nature.com! Check it out here along with @mattdv-t.bsky.social’s ‘behind the paper’ article!
17.02.2026 18:55 — 👍 25 🔁 6 💬 5 📌 0
@natcomms.nature.com
26.11.2025 15:36 — 👍 0 🔁 0 💬 0 📌 0
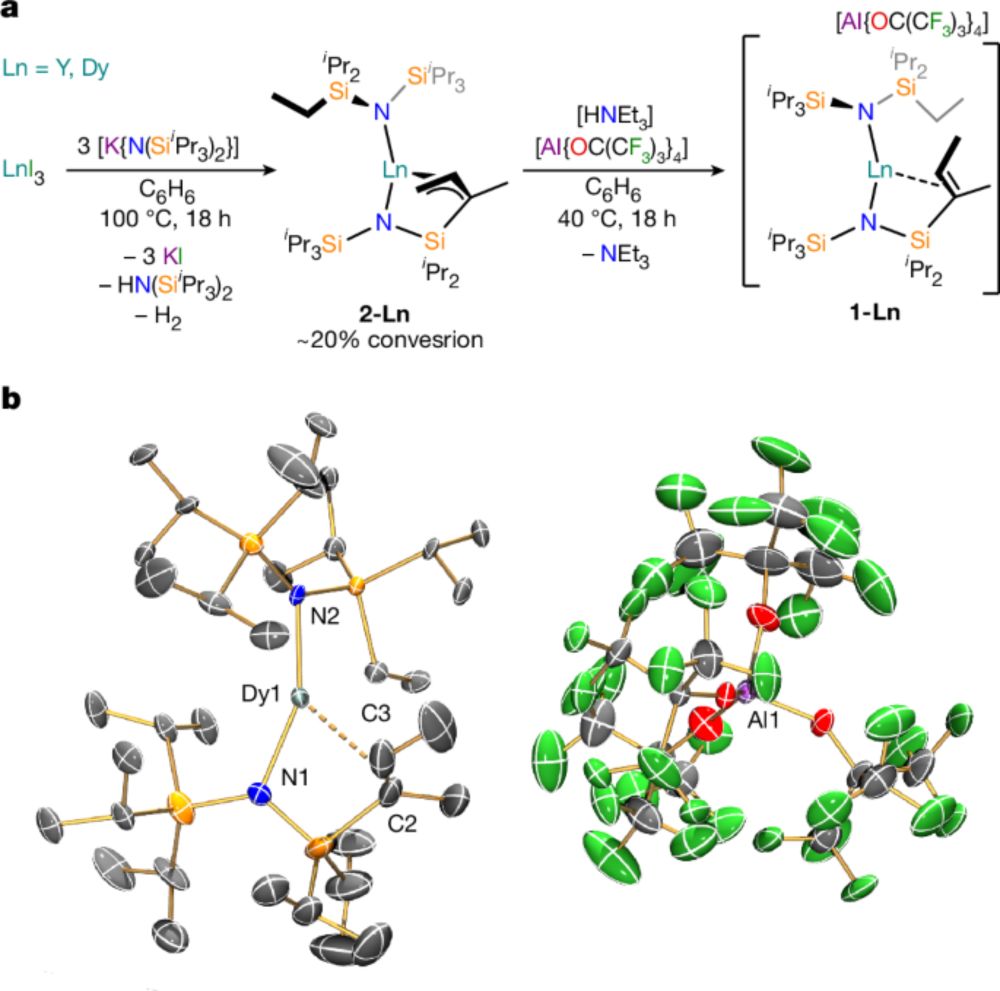
@john-seed.bsky.social collected some gorgeous magnetic data - as always! In fact the Tb(III) data suggested a well-isolated paramagnetic pseudo-doublet spin-orbit ground state which has led to some spin-off work so stay tuned!
21.10.2025 08:03 — 👍 2 🔁 1 💬 1 📌 0
Huge (!) congratulations to Becky and Jingzhen for some tremendous synthetic chemistry, and for the former's 1st first author publication, and to all authors who helped contribute to this work - especially @haenisch-group.bsky.social at @unimarburg.bsky.social. Please read and enjoy!
15.08.2025 16:25 — 👍 0 🔁 0 💬 1 📌 0
Thanks a lot for the support Dave! 😄
17.07.2025 17:00 — 👍 1 🔁 0 💬 0 📌 0
Thank you!
17.07.2025 17:00 — 👍 0 🔁 0 💬 0 📌 0
I think I’ll be using that analogy from now on. Thanks a lot - glad you like it!
10.07.2025 20:12 — 👍 1 🔁 0 💬 0 📌 0
Huge congratulations Dave, Nick, Gemma and team! 🎉🥳
26.06.2025 16:40 — 👍 2 🔁 0 💬 1 📌 0

Breaking Bonds at Tin(II): Reductive or Oxidative Addition?
Here, the addition of H–H, Be–Be, B–B, and B–H bonds to SnII is studied by experimental and theoretical means. We report the first examples of B–B and Be–Be bond additions to a main group metal centr...
Breaking bonds at tin(II)! We've had a look at the spectrum of bond addition reactions, from reductive (Be-Be) to oxidative (Cl-Cl), and everything in between (B-B, B-H, H-H)
@angewandtechemie.bsky.social @maxdietz.bsky.social @amelia-swarbrook.bsky.social
doi.org/10.1002/anie...
02.06.2025 19:53 — 👍 31 🔁 4 💬 0 📌 2
Good luck with everything Gemma!
30.05.2025 22:44 — 👍 1 🔁 0 💬 0 📌 0
Trithorium superatom confounds with its unexpected and ‘mind-blowing’ diamagnetism
Nanocluster behaves like a 'super alkali-metal'
The first superatoms made using actinides ever created are adding a new dimension to one of the strangest chemical phenomena.
#ChemSky
06.05.2025 14:40 — 👍 12 🔁 2 💬 0 📌 0
Thanks a lot Lisa. Glad you like it!
17.04.2025 20:31 — 👍 0 🔁 0 💬 0 📌 0
Thanks so much! 😃
17.04.2025 20:31 — 👍 0 🔁 0 💬 0 📌 0
Thanks Andrew. Absolutely! 🤝🏻
17.04.2025 20:30 — 👍 1 🔁 0 💬 0 📌 0
This has been a mountain of work from all authors, and impossible without the contributions of the whole team. Eternally grateful to have such talented, knowledgeable, and collaborative colleagues here in Manchester. Please read and enjoy! @uniofmanchester.bsky.social
11.04.2025 12:22 — 👍 0 🔁 0 💬 0 📌 0
However, in external applied magnetic fields, these S = 1/2 clusters are diamagnetic (!) as the valence delocalisation enables exalted diamagnetism, which overwhelms the intrinsic paramagnetic character, experimentally evidencing actinide open-shell superatomic character.
11.04.2025 12:22 — 👍 0 🔁 0 💬 1 📌 0
Inorganic Radical Chemistry🧪
Final Year PhD in the Mehta Group 🏴
Inorganic chemistry research group at King’s College London 🧪 organometallic & main group content
PDRA, Bakewell Group at KCL (formerly: PDRA, Willcox Group, Manchester; PhD, Stasch Group, St Andrews). Main Group inorganic chemist, loves pretty crystals, sometimes posts pictures of food
Associate Professor of Inorganic Chemistry @westernu.ca 👨🔬 🇨🇦 | Organometallic & Main Group Chemistry 🧪
Group webpage: www.droverlab.ca/publications
12th International Conference on f-Elements
The University of Manchester - 2026
Visit: www.ICFE12.manchester.ac.uk
Professor of Mineralogy at University of Bristol, HMG Advisor. Aspirational TV presenter. Step-parent. Trail runner / squelcher. Specialises in geological disposal of radioactive waste.
█▓▒░░organometallics░░▒▓█ https://kilpatrickgroup.uk
See also research group account: @kilpatrickgroup.bsky.social
Nature Communications is an open access journal publishing high-quality research in all areas of the biological, physical, chemical, clinical, social, and Earth sciences.
www.nature.com/ncomms/
Professor of chemistry at the EPFL, Switzerland
lcs.epfl.ch
Organometallic Chemist | Postdoctoral Researcher at FAU Erlangen-Nürnberg
PDRA/Beamline scientist | f-block Chemist | X-ray spectroscopist | software developer
On a particular mission to develop beamline sample environments, data acquisition and treatment methods, and computational characterisation tailored for actinides.
Senior Lecturer in Inorganic and Computational Chemistry at the University of Bath
https://people.bath.ac.uk/cm2025/index.html
Heisenberg-Professor in inorganic chemistry at Leipzig University (Germany). Less is more: low-coordinate and low-valent chemistry of 3d metals.
Doctoral student at EPFL🇨🇭in coordination f-element chemistry
Final year PhD student in the Crimmin group at Imperial College London
Nice spectroscopy and so forth. Quantitative electronic structure insights. Michael L. Baker research group, University of Manchester.
mlbakerlab.co.uk