Shmeta-analysis.
05.06.2025 18:06 — 👍 2 🔁 1 💬 1 📌 0@kaulcsmc.bsky.social
Cardiologist, evidence appraiser, data detective, rock music fan
@kaulcsmc.bsky.social
Cardiologist, evidence appraiser, data detective, rock music fan
Shmeta-analysis.
05.06.2025 18:06 — 👍 2 🔁 1 💬 1 📌 0Post-treatment discontinuation eGFR trajectory is somewhat reassuring. No?
05.06.2025 16:08 — 👍 1 🔁 0 💬 1 📌 0I think it is a positive first step, but whether there is a convincing evidence of an additive effect of combination therapy requires proof in an outcome study.
For now, combination therapy has won the bragging rights, but I would not go so far as to proclaim this is a practice-changing trial.
Is there a dose-response or a threshold relationship with UACR reduction and clinical outcome benefit? In other words, will a >50% reduction translate into a greater kidney or CV benefit than >30% reduction?
05.06.2025 14:59 — 👍 3 🔁 0 💬 1 📌 0Hyperkalemia on combo vs nsMRA: RR 0.82, 95% CI 0.50-1.36
No clearcut evidence that hyperkalemia risk is mitigated by combining sglt2i with nsMRA (? Inadequate power/Sample Size)

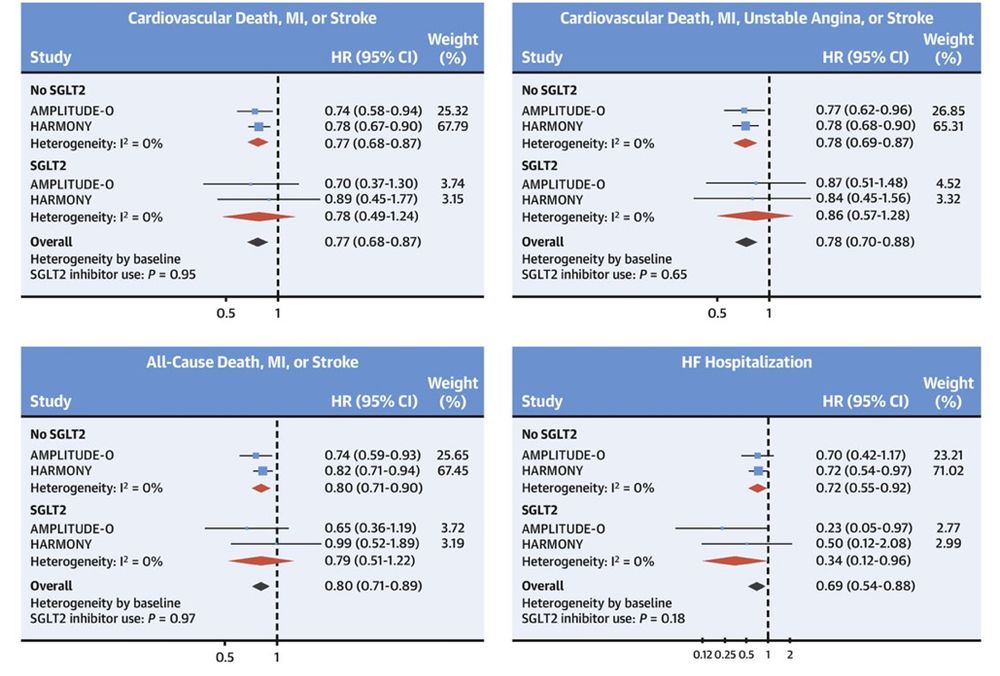
No 'additive' cardioprotective effect of GLP-1RA on background use of SGLT2i in HARMONY, AMPLITUDE-O and most recently SOUL.
Cardioprotective effects of GLP-1RA are 'independent' of SGLT2i
ahajournals.org/doi/epdf/10.11…
10.1016/j.jacc.2023.05.048
Pragmatic trials like comparative effectiveness studies require realistically ‘sober’ assumptions to account for the issues you stated.
Lipid hypothesis lingered on for several decades until LDL-C lowering was proven to reduce CV risk. Analogy applies to INOCA/ANOCA/MINOCA. Need novel targeted Rxs.
95% CI is compatible with a 6% benefit (clinically trivial) to 37% harm (clinically important). If CI contained a clinically important benefit, say HR of 0.80 or a more plausible HR of 0.85 to 0.90, but CI was wide spanning across HR of 1, then an inference of inconclusive result would be justified.
30.03.2025 23:16 — 👍 1 🔁 0 💬 1 📌 0Trial powered for a HR of 0.80 which was excluded by the 95% CI.
Is it still a null outcome?
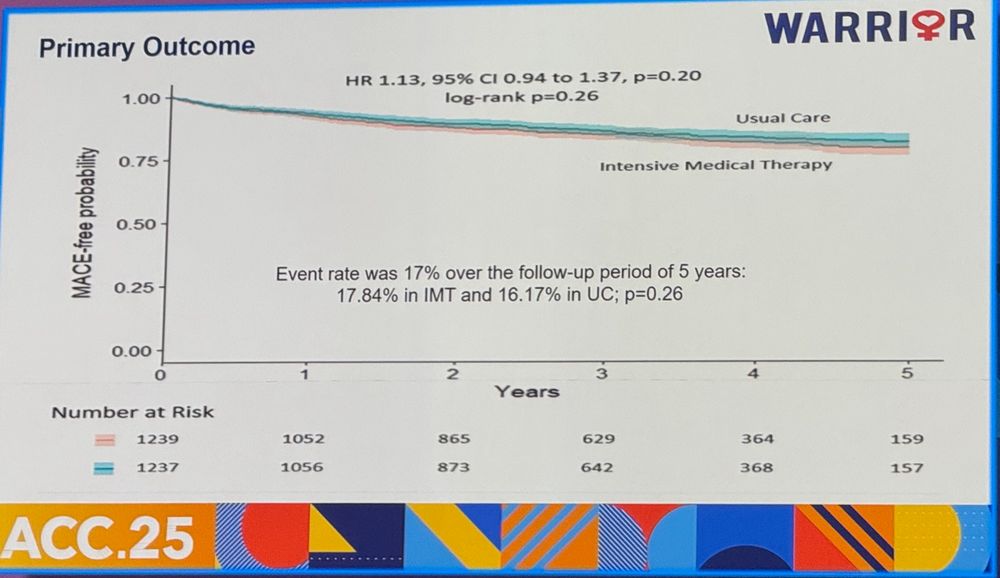
Warrior trial PEP result shown below.
Is this a NULL trial or a NEGATIVE trial?
Trial powered for HR 0.80 which was excluded by 95% CI
How many null or negative PRAGMATIC trials before we seriously consider/scrutinize the utility of such trials?

Very proud of this work with @kaulcsmc.bsky.social just published in Progress in Cardiovascular Diseases
Are ORBITA trials practice-changing?
We discuss whether the enthousiasm of some after ORBITA-2 is really justified.
www.sciencedirect.com/science/arti...
#medsky
#cardiosky

Snake oil salesmen of the 21st century!
Here they come.
endpts.com/rfk-jr-conve...
Are the Device Approval Standards Lax? JACC Cardiovasc Interv. 2025 Feb 10;18(3):380-384. doi: 10.1016/j.jcin.2024.08.037. PMID: 39939040.
Device Approval in the United States: The FDA Is on Target. JACC Cardiovasc Interv. 2025 Feb 10;18(3):385-387. doi: 10.1016/j.jcin.2024.10.044. PMID: 39939041.
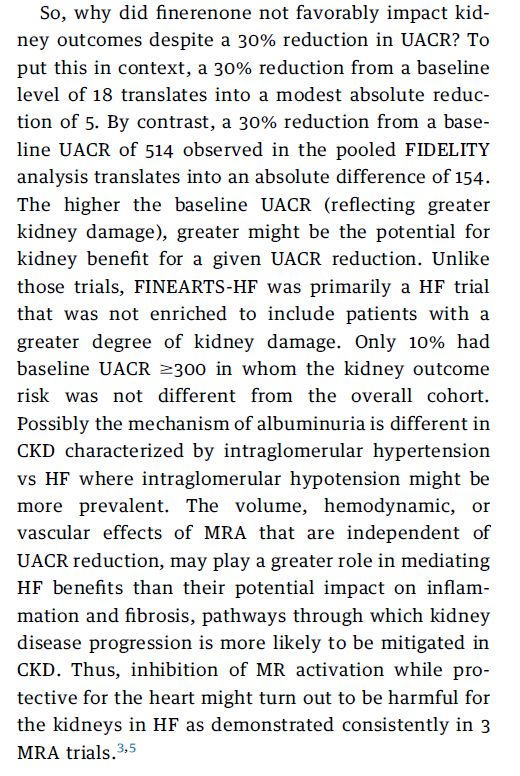
So, why did finerenone not favorably impact kidney outcomes despite a 30% reduction in UACR? To put this in context, a 30% reduction from a baseline level of 18 translates into a modest absolute reduction of 5. By contrast, a 30% reduction from a baseline UACR of 514 observed in the pooled FIDELITY analysis translates into an absolute difference of 154. The higher the baseline UACR (reflecting greater kidney damage), greater might be the potential for kidney benefit for a given UACR reduction. Unlike those trials, FINEARTS-HF was primarily a HF trial that was not enriched to include patients with a greater degree of kidney damage. Only 10% had baseline UACR $300 in whom the kidney outcome risk was not different from the overall cohort. Possibly the mechanism of albuminuria is different in CKD characterized by intraglomerular hypertension vs HF where intraglomerular hypotension might be more prevalent. The volume, hemodynamic, or vascular effects of MRA that are independent of UACR reduction, may play a greater role in mediating HF benefits than their potential impact on inflammation and fibrosis, pathways through which kidney disease progression is more likely to be mitigated in CKD. Thus, inhibition of MR activation while protective for the heart might turn out to be harmful for the kidneys in HF as demonstrated consistently in 3 MRA trials.3,5
Editorial on the renal results of FINEARTS HF in @jaccjournals.bsky.social from @kaulcsmc.bsky.social and moi
Free link:
authors.elsevier.com/a/1kQ%7Ec2d9...
#NephSky #CardioSky #Finerenone
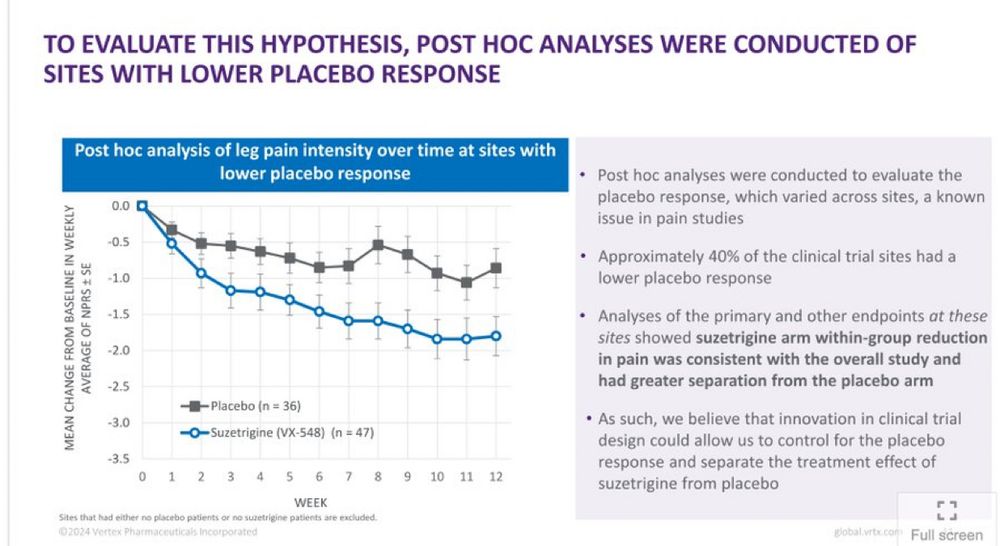
Imagine if I excluded centers in a multicenter trial of SAVR vs TAVR where TAVR outperformed SAVR, I could show that SAVR was superior to TAVR in high-risk patients with AS (PARTNER-1A).
19.12.2024 19:48 — 👍 6 🔁 2 💬 2 📌 0Should Heart Failure Trials routinely report on all-cause hospitalizations?
Should clinicians, guidelines & payers favor interventions that favorably impact cause-specific & all-cause outcomes?
jamanetwork.com/journals/jaman…
jamanetwork.com/journals/jaman
Who are the optimal candidates for acoramidis therapy?
When should it be considered first-line therapy?
Does the proposed cost provide value?

As expected!
www.statnews.com/2024/11/22/b...
NI margin in EVEREST II trial of MitraClip vs MV surgery for MR was 31% absolute difference, a margin through which one could drive 3 buses lined side by side.
Trial met NI despite the fact that HR excluded 1.0, i.e., MitraClip was INFERIOR to surgery, yet NONINFERIOR!
www.nejm.org/doi/full/10....
Even though the observed rates were lower than assumed (22%), had NI margin been fixed as risk ratio of 1.18 ([22+4]/22), the overall results would still have met NI as RR was 0.90, 0.77-1.05. An excellent example of how a NI trial should be designed and executed. Congrats to trial investigators.
22.11.2024 02:17 — 👍 1 🔁 1 💬 0 📌 0So you are suggesting you need longer f/u and greater LDL and Lp(a) reduction, both of which favor PREVAIL. So what is the probability of success?
20.11.2024 21:20 — 👍 0 🔁 0 💬 1 📌 04/
Key Qs
What is probability of success with obicetrapib?
Why should obicetrapib succeed when 2 other CETPi did not?
Is absolute or relative LDL reduction key driver of benefit?
Is duration of treatment important?
Is LDL lowering with CETPi different from other LDL lowering Rx?
3/
PREVAIL(Obicetrapib), N=9541, F/U: ~4y
Mean LDL 103; LDL⬇️35-40%, Lp(a)⬇️45% (based on BROOKLYN)
PEP (4-MACE): Powered for 15-20% RRR
Recruitment completed; event-driven trial
Targeting higher baseline LDL & higher risk patients
2/
REVEAL (Anacetrapib), N=30,449, F/U: 4.1y
Mean LDL 61; LDL⬇️41%, Lp(a)⬇️25%
PEP (3-MACE): 10.8% vs 11.8%, HR 0.91, 0.85-0.97
Development halted (long half-life, modest Rx effect)
1/
Is LDL-C lowering by CETP inhibitor cardioprotective?
Brief history
ACCELERATE (Evacetrapib), N=12092, F/U: 28m
Mean LDL 81; LDL⬇️37%, Lp(a)⬇️22%
PEP (MACE-5): 12.9% vs 12.8% HR 1.01, 0.91-1.11
Trial stopped early due to futility
x.com/mdavidsonmd/...
Nonetheless, the clinical relevance of the favorable, but modest, MRI changes induced by Tirzepatide remain unclear.
19.11.2024 18:54 — 👍 1 🔁 0 💬 0 📌 0What this graph tells us is that about 12% (R squared) of variability in LV mass can be explained by weight loss, thereby indicating that reduction in LV mass might be related to a possible direct cardiac effect or a paracrine effect,
possibly modulated via paracardiac adipocytes.
But need to know which criteria for CRNM bleed was driving it? There were 3 or 4 criteria specified? A visit to hospital or ER is more relevant than some other ones.
16.11.2024 21:16 — 👍 0 🔁 0 💬 1 📌 07/
So, what to make of a trial with 50% lower events than expected (sum total of 85 events over 3y in a trial enrolling 1600 patients), lots of missing data, & biased choice of safety outcome?
Enthusiasts of LAAC will celebrate 'win', but should clinicians & payers join in?
6/
But wait, should safety outcome not have captured procedural bleeding which is⬆️with LAAC? Had ISTH major bleed outcome (including procedural bleeding) been used, superiority would not have been met, and hence the win criterion would not have been met, strictly speaking!