Thanks for being part of it Melanie😊
03.09.2025 05:27 — 👍 1 🔁 0 💬 0 📌 0Jeremy Garb
@garbjeremy.bsky.social
PhD student at the Sorek lab. Immune proteins.
@garbjeremy.bsky.social
PhD student at the Sorek lab. Immune proteins.
Thanks for being part of it Melanie😊
03.09.2025 05:27 — 👍 1 🔁 0 💬 0 📌 0Thank you Jens😊
02.09.2025 14:17 — 👍 1 🔁 0 💬 0 📌 0Thanks François
02.09.2025 14:17 — 👍 0 🔁 0 💬 0 📌 0I had so much fun making this project come to life!
Huge thanks to everyone who made it happen 💙 - especially my brilliant collaborators
David W. Adams, Eliane Hadas Yardeni, @mblokesch.bsky.social, and of course @soreklab.bsky.social
Our work shows that synthetic proteins can be designed to defeat bacterial immune systems.
This strategy could:
✅ Broaden the host range of therapeutic phages.
✅ Allow personalized phages for each patient’s infection.
✅ Unlock genetic engineering in “untouchable” bacteria for biotech + medicine.

It’s not just phages either.
We designed inhibitors for DdmDE, a system in Vibrio cholerae that usually clears plasmids from transformed cells.
Two of these binders conferred the plasmids with significant resistance against DdmDE-mediated plasmid clearance even in its native bacterial strain.

Now that we had binders against two defense systems, we engineered a single phage to carry two inhibitors.
➡️ This phage could overcome two defenses in the same bacterium.
This shows we can build “multi-resistant” phages for therapy.

We also built inhibitors against Avs1, a bacterial ancestor of human immune NLRs.
Synthetic binders blocked Avs1 activity—again, letting phages replicate in bacteria that co-express our binders with Avs1.

Using this workflow, we successfully identified multiple binders that inhibit Thoeris when co-expressed during infection ✅
02.09.2025 07:36 — 👍 0 🔁 0 💬 1 📌 0
To test candidate binders, and check if they successfully inhibit anti-phage defense systems, we built a 96-well transformation and phage-infection workflow (otherwise – far too many transformations!). This let us screen ~200 candidates at a time against a given system.
02.09.2025 07:36 — 👍 0 🔁 0 💬 1 📌 0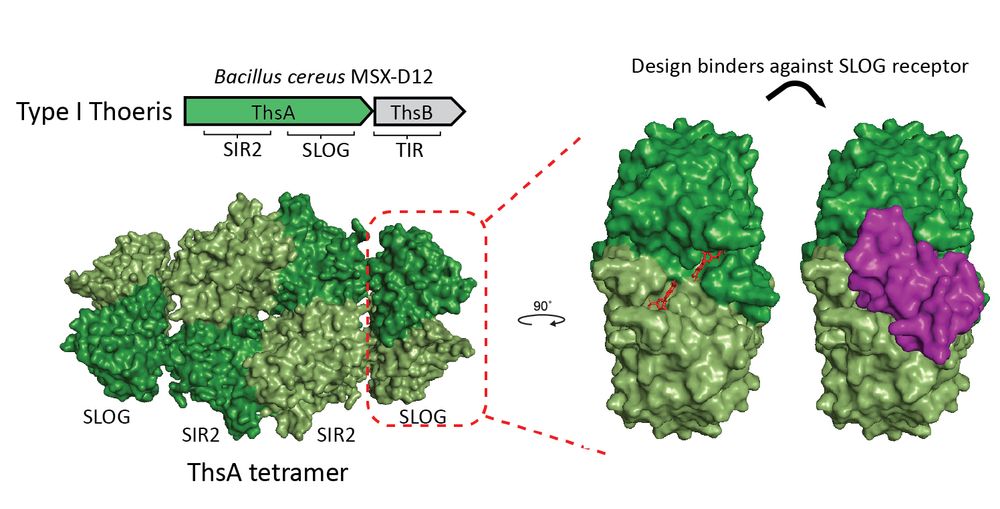
We started with the Thoeris system, where ThsB makes a signal (3′cADPR) that activates ThsA by binding its SLOG receptor domain.
We hypothesized that designing small proteins that bind the molecule-sensing site in the SLOG domain would disrupt its ability to perceive the signaling molecules.
What if we design proteins to disable these defenses?
Using AI-driven de novo protein design (RFdiffusion), we created small proteins (49–98 aa) that bind and block bacterial defense proteins 💻🤖🧬
Some background 📖
Bacteria are far from defenseless.
They carry dozens of anti-phage and anti-plasmid systems—CRISPR is just the tip of the iceberg.
➡️ This makes phage therapy often fail in patients.
➡️ It also makes many bacteria very hard to genetically engineer.

📢 New preprint alert!
We designed synthetic proteins that can block bacterial immune systems, allowing phages + plasmids to overcome natural defenses.
This could transform phage therapy + genetic engineering.
Here’s what we found 🧵
Preprint🔗: www.biorxiv.org/content/10.1...
@dinahoch.bsky.social dingazzz
16.11.2024 19:25 — 👍 3 🔁 0 💬 0 📌 0