We are finishing eval for a model with better capabilities in this regard and I'm excited to test this case within this one to see if this behavior is resolved
14.10.2025 13:51 — 👍 0 🔁 0 💬 0 📌 0@openevidence.bsky.social
@openevidence.bsky.social
We are finishing eval for a model with better capabilities in this regard and I'm excited to test this case within this one to see if this behavior is resolved
14.10.2025 13:51 — 👍 0 🔁 0 💬 0 📌 0"makes sense" and "desired behavior" are of course different things. I think this is a great example of how ambiguity in query can trip us up where better self reflection after citation retrieval could help. Basically, which interpretation of the Q makes most sense AFTER we pull references.
14.10.2025 13:51 — 👍 1 🔁 0 💬 1 📌 0
There should be a share icon in top right. We make you attest that there is no PHI in the query before you make it public (see below) then you should be able to copy link
14.10.2025 13:47 — 👍 0 🔁 0 💬 1 📌 0Yeah I think there is something going on with being confused whether "dual" refers to ETA and ETB or refers to ETA and angiotensin. At the end of the answer you posted it used dual to talk about ETA and ETB. Thanks for posting.
14.10.2025 13:22 — 👍 0 🔁 0 💬 0 📌 0No this isn't right.... I see now from your answer it was sending you experimental ET_A antagonists... Ok. Well we will dig and figure out what's going on
14.10.2025 13:19 — 👍 0 🔁 0 💬 1 📌 0Ok I THINK I know what's going on. I believe it's interpreting "dual endothelin" and a request for a inhibitor of both ETA and ETB, but sparsentan only blocks ETA. If I can get the raw query, we will dive into it on our quality team and figure it out
14.10.2025 13:18 — 👍 1 🔁 0 💬 2 📌 0
Would you mind sharing the link to the answer. I cant replicate. Always looking to improve
www.openevidence.com/ask/21690269...

@travizack.bsky.social and Varun from OE talk AI and the future of medical information on the STAT-AI podcast:
open.spotify.com/episode/121y...
We have a new deepconsult feature that is a bit more directed at literature review.
04.07.2025 14:41 — 👍 2 🔁 0 💬 0 📌 0Happy first day!
To all interns, residents, fellows, and new attendings.
We are building specialty specific learning communities at OE. If you are interested in joining peers and learning together with Openevidence, shoot us an email at md_community@openevidence.com

Dr. Atul Butte was laid to rest today. His large shoulders and larger vision have been foundational to all AI can and will accomplish in healthcare.
His family is requesting those interested to please consider supporting research on mpnst here: giving.ucsf.edu/fund/maligna...

Selfie of Lancet Senior Executive Editor, Vania Wisdom, and Acting Deputy Editor at The Lancet Oncology, Kat Gourd. Both are Lancet Oncology Ambassadors, attending the American Society of Clinical Oncology (ASCO) conference in Chicago, USA.
👋 Our editors are on-site at #ASCO25, Chicago.
We’re excited to be part of this gathering of global cancer experts—come & meet us.
‘Contact the Editor’ details below, in-thread 🧵 @ascocancer.bsky.social

Hello from the City of Lakes! We’re thrilled to welcome thousands of members of the ob-gyn community to the 2025 Annual Clinical & Scientific Meeting. From the heart of the Midwest, we’re kicking off a weekend of connection, learning, and inspiration. Share the excitement using #ACOG2025!
15.05.2025 22:01 — 👍 9 🔁 2 💬 1 📌 1
As for the safety, I asked @openevidence.bsky.social which is a chatbot trained on the professional medical literature. The current thinking is that it is not ready for primetime. www.openevidence.com/ask/2ca4fd30...
13.05.2025 01:00 — 👍 2 🔁 2 💬 1 📌 0Welcome JAMA!!
13.05.2025 01:26 — 👍 1 🔁 0 💬 0 📌 0
we are now offering free CME credits just by OE in yuour normal practice:
www.openevidence.com/announcement...
I don't know... How do you feel :)
17.04.2025 22:44 — 👍 0 🔁 0 💬 0 📌 0Congratulations 🎉 excited to welcome them to UCSF family
21.03.2025 19:47 — 👍 0 🔁 0 💬 0 📌 0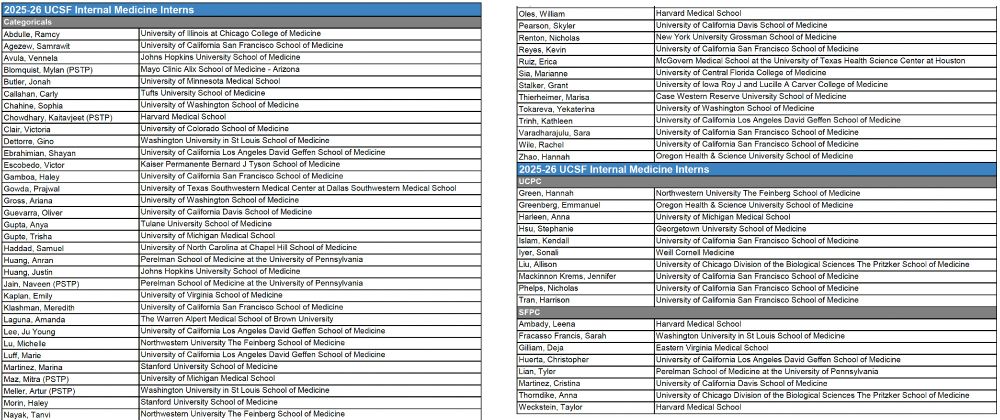
So thrilled to welcome this extraordinary group of 64 young physicians to @UCSF and @UCSFimresidency. A great day!
(UCPC=UCSF Health-based primary care program; SFGH=San Francisco General-based primary care; PSTP=physician-scientist training program) @UCSFIMChiefs @UCSFDOM

@travizack.bsky.social and Varun from OE talk AI and the future of medical information on the STAT-AI podcast:
open.spotify.com/episode/121y...
www.openevidence.com/ask/88013027...
15.02.2025 01:26 — 👍 0 🔁 0 💬 0 📌 0
That's really amazing! Hope this helps
www.openevidence.com/ask/47b4d834...
Some background on current limited TX options:
www.openevidence.com/ask/a94c01ac...
However, there is a recently opened clinical trial for this rare disease
clinicaltrials.gov/study/NCT061...
@jerryclee.bsky.social
Some mechanistic background:
www.openevidence.com/ask/07ad3d33...
A short review of where we were at prior to this trial
www.openevidence.com/ask/1e95f6d9...
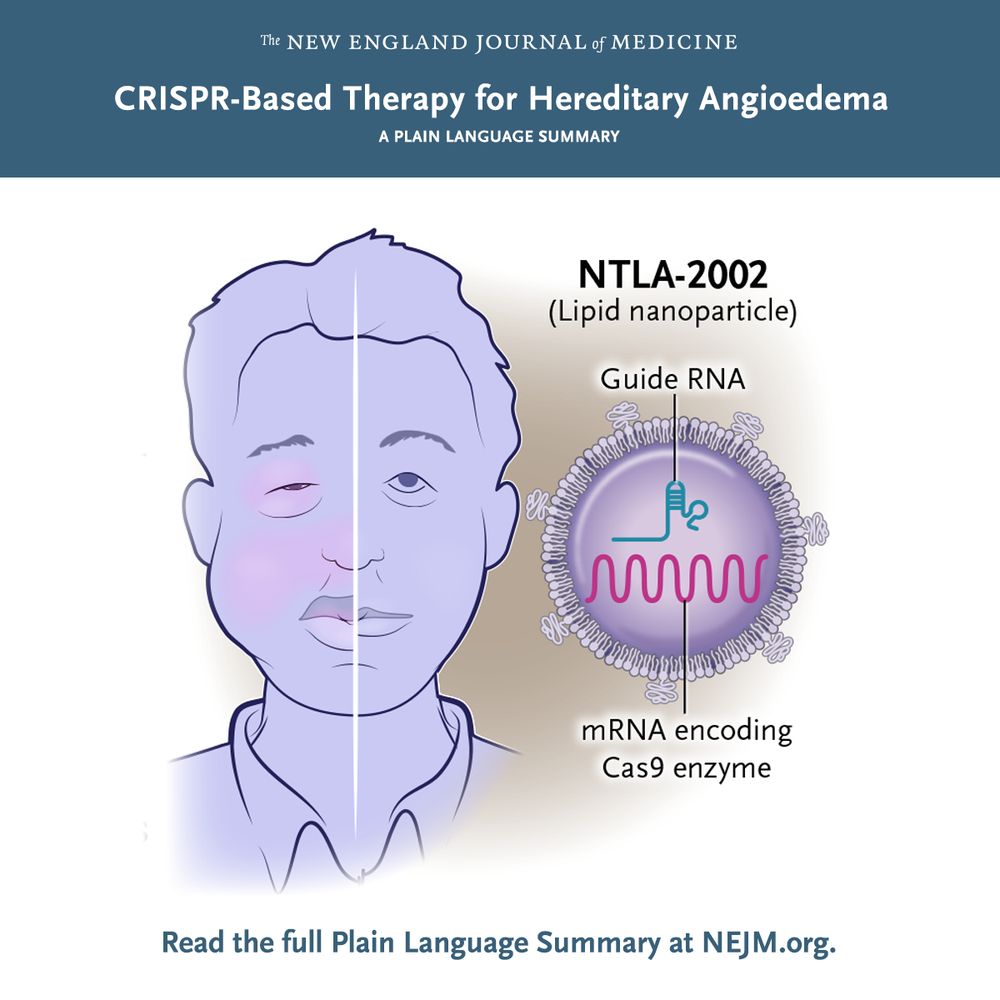
The New England Journal of Medicine CRISPR-Based Therapy for Hereditary Angioedema A PLAIN LANGUAGE SUMMARY Illustration of a patient with hereditary angioedema and NTLA-2002, a nonviral gene-editing therapy based on CRISPRCas9, for treatment. Read the full Plain Language Summary at NEJM.org.
In a new phase 2 trial, researchers evaluated the efficacy of a gene-editing therapy, NTLA-2002, in preventing angioedema attacks in patients with hereditary angioedema. Full trial results and Plain Language Summary: nej.md/4heR7hT
#MedSky #Oncology
www.openevidence.com/ask/55b54926...
05.02.2025 02:34 — 👍 0 🔁 0 💬 0 📌 0As a primary care doctor & clinical informatician, I love using @openevidence.bsky.social during my clinic visits to review latest guidelines with patients & sources.
I strongly believe this practice helps build stronger therapeutic relationship with the doctor + AI assisted CDS. #CI=AI
Thanks so much for the shoutout. Ask for a patient summary. We will provide the information at an appropriate and inclusive reading level and can accommodate many languages
05.02.2025 02:27 — 👍 1 🔁 0 💬 1 📌 0