Our U-CARE Venue 2025 film is here! 🎬
Catch highlights from this year’s conference, themed ‘Smarter Science – How can we conduct high-quality healthcare research more efficiently?’
Watch the 1-minute video, featuring Shaun Treweek ✨
@streweek.bsky.social
#UCAREVenue2025 #UCARE
24.11.2025 11:48 — 👍 3 🔁 1 💬 0 📌 0

A screenshot of the website linked to from the LinkedIn post.
A fully funded, equity-related PhD on offer at the U-CARE, Uppsala Trial Forge Centre, Sweden:
lnkd.in/eW_HhtKm
The PhD is about working with clinical trial and population register datasets related to equity, diversity, and inclusion.
Deadline is 29th Dec 2025.
@u-care.bsky.social
03.12.2025 10:48 — 👍 1 🔁 0 💬 0 📌 0
5/5 We are long over due it being time to act to answer Doug Altman's call from 31 years ago for "less research, better research, and research done for the right reasons".
www.bmj.com/content/308/...
trialsjournal.biomedcentral.com/articles/10....
@streweek.bsky.social
24.09.2025 09:24 — 👍 1 🔁 1 💬 0 📌 0

Don't miss out on your chance to attend this years #Edinburgh #ClinicalTrial Management Course on 06 & 07 Nov 2025- Register here edin.ac/3RGb0mB #ECTMC25 @streweek.bsky.social @bleedingstroke.bsky.social @ace-aberdeen.bsky.social @merrij.bsky.social
22.10.2025 14:32 — 👍 2 🔁 1 💬 0 📌 0

Who is in your trial? Improving the reporting of participant characteristics in trial protocols and results - Trials
From 1st Jan 2026 the journal Trials will mandate that authors present information about the age, sex, gender, ethnicity, socioeconomic status and geographical location of expected and actual participants in their protocols and trial results.
trialsjournal.biomedcentral.com/articles/10....
28.10.2025 08:37 — 👍 1 🔁 0 💬 0 📌 0

📣 Our @streweek.bsky.social is lead author on an editorial in #Trials outlining a new mandate in the journal - mandating the reporting of six key participant characteristics in any protocol/trial report submitted to Trials on or after 1st Jan 2026. 1/2
trialsjournal.biomedcentral.com/articles/10....
15.09.2025 11:24 — 👍 6 🔁 6 💬 1 📌 0
I'm unsure. But evaluating what we do seems sensible. And perhaps we should bundle things a bit more in SWATs so that we evaluate a broader strategy rather than a single component of strategy, sometimes at least.
12.09.2025 09:05 — 👍 0 🔁 0 💬 0 📌 0

Who is in your trial? Improving the reporting of participant characteristics in trial protocols and results - Trials
From 1st Jan 2026, the journal Trials will mandate reporting of (at least) six participant characteristics for both protocols and reports of trial results. Read more in the editorial:
trialsjournal.biomedcentral.com/articles/10....
@ukcrc-ctu.org.uk @ecrin.bsky.social
12.09.2025 08:58 — 👍 8 🔁 3 💬 0 📌 0

Picture of Shaun thanking people supporting health and care research in 2025 as part of Red4Research Day 2025.
It's Red4Research Day today!
Thanks to everyone – research participants, patients, professionals, volunteers and regulatory bodies – working together to do health and social care research.
rdforum.nhs.uk/red4research...
#Red4Research
20.06.2025 07:31 — 👍 9 🔁 1 💬 2 📌 0
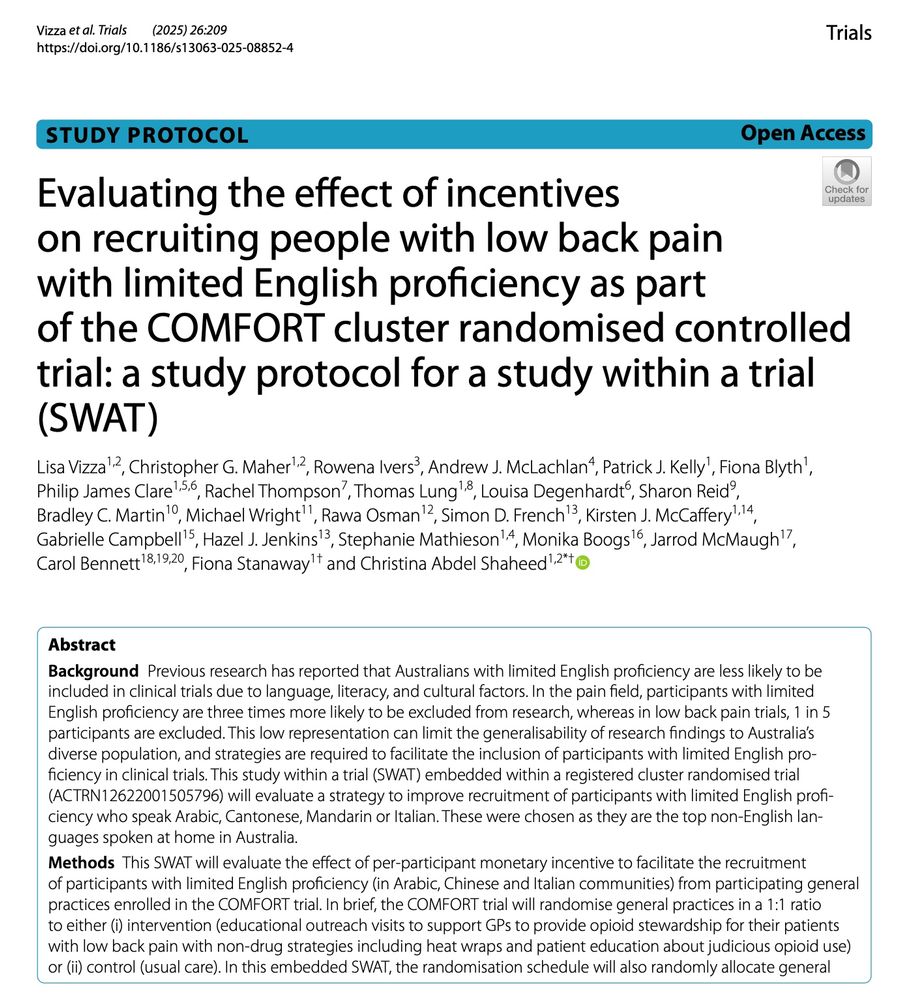

A couple of Studies Within A Trial (SWATs), one results, one a protocol, just out in the journal Trials:
trialsjournal.biomedcentral.com/articles/10....
trialsjournal.biomedcentral.com/articles/10....
19.06.2025 16:07 — 👍 1 🔁 0 💬 0 📌 0
🎉 Stage 2 is LIVE - where YOU get to decide the People's favourite question!
➡️ If you're joining The People's Review for the first time sign-up here: buff.ly/kAuYITA
➡️ If joined us for Stage 1 you can login in directly to Cochrane Crowd here: buff.ly/q0WudTM
20.05.2025 19:38 — 👍 4 🔁 5 💬 1 📌 1

The Edinburgh Clinical Trial Management Course flyer, the details on it are available at the link in the post.
The Edinburgh Clinical Trial Management Course, Nov 6th and 7th 2025 in Edinburgh, is now open for bookings:
clinical-research-facility.ed.ac.uk/edinburgh-cl...
08.05.2025 08:49 — 👍 1 🔁 0 💬 0 📌 0

We’re bombarded with health advice 24/7. Systematic reviews help cut through the noise—but most people don’t know what they are.
💥 That’s where #ThePeoplesReview comes in. Learn about systematic reviews, by doing a systematic review
🔗https://www.thepeoplesreview.ie/join-now
06.05.2025 15:50 — 👍 2 🔁 0 💬 0 📌 0
To kick off #MethodologyMonday in May, we bring you a poignant article by the Editors in Chief of the Journal of Clinical Epidemiology. They address the “elephant in the room”, the upheaval of the global evidence research eco-systems pursued by the US administration.
www.jclinepi.com/article/S089...
05.05.2025 13:21 — 👍 7 🔁 5 💬 1 📌 0

We spoke with @streweek.bsky.social about why so much health research still falls short. Shaun argues that big parts of health research is “bad” & does not bring the needed value to the evidence-base. Tune in to learn how we can conduct “good” research for our patients 🎙️!
28.04.2025 10:56 — 👍 2 🔁 1 💬 0 📌 1

💻 Come along to our next Capacity Strengthening Hub webinar with @tghn-news.bsky.social to hear about experts' experience, general principles and a practical framework for communicating #ClinicalTrials results with participants.
🗓️ 2 June
🕙 10:00-11:30 BST
📍 Online
Register now: bit.ly/4k28ALd
05.05.2025 11:02 — 👍 2 🔁 3 💬 0 📌 0

A picture of the webpage that you get to at the link. It shows lots of different types of people.
We’re bombarded with health advice 24/7. 😵💫 Systematic reviews help cut through the noise—but most people don’t know what they are.
💥 That’s where #ThePeoplesReview comes in. Learn about systematic reviews, by doing a systematic review
www.thepeoplesreview.ie
22.04.2025 16:30 — 👍 3 🔁 0 💬 1 📌 0
This literature review identified strategies to enhance the racial & ethnic diversity of breast cancer trial populations. It resulted in 8 key strategic themes, which were used to create a new Racial and Minority Growth model.
#MethodologyMonday
Scott & Westwell
orca.cardiff.ac.uk/id/eprint/17...
21.04.2025 06:13 — 👍 1 🔁 1 💬 0 📌 0
A major update to the CONSORT reporting guidance for clinical trials was published last week.
📌 CONSORT 2025 replaces all previous versions and should be used from now on.
So what’s new and what’s different? 1/7
#MethodologyMonday #116
(COI - I am a co-author)
21.04.2025 06:49 — 👍 35 🔁 27 💬 2 📌 1
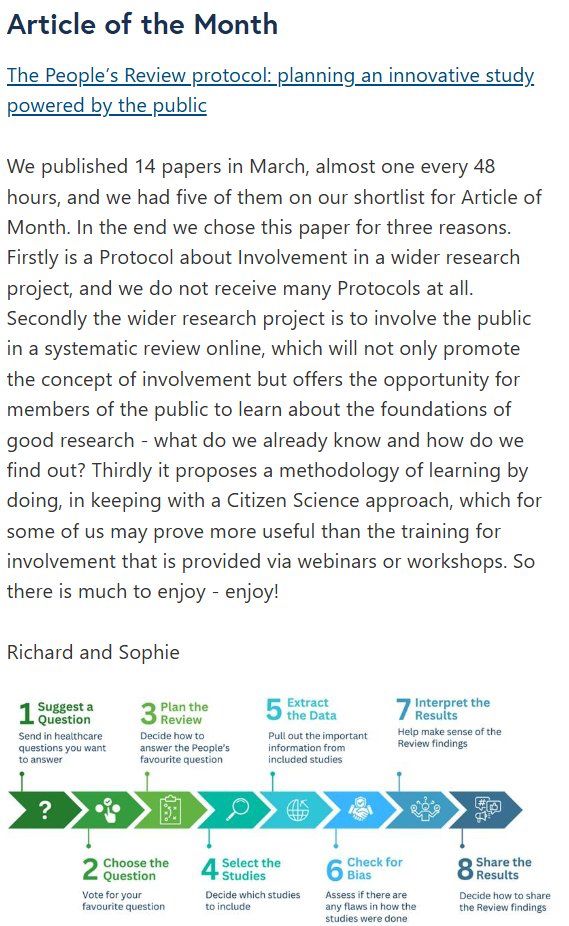
🚨We are thrilled that our protocol has been selected as the article of the month in Research Involvement and Engagement.
Full article here: researchinvolvement.biomedcentral.com/articles/10....
#ThePeoplesReview #PoweredByThePublic #ArticleOfTheMonth
14.04.2025 14:14 — 👍 5 🔁 6 💬 1 📌 0
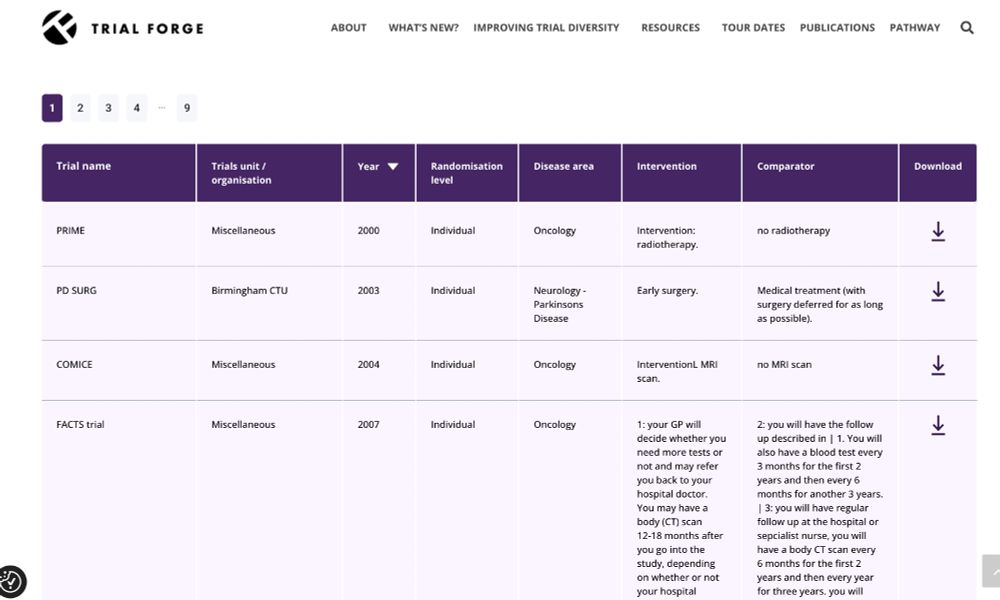
We have just created a library of participant information leaflets for trials, over 200, if you are looking for inspiration.
Check out:
www.trialforge.org/excelsior-pi...
The is part of EXCELSIOR, a project ed by Frances Shiely at Cork University.
11.04.2025 07:58 — 👍 5 🔁 3 💬 0 📌 0
Many thanks! I hope that you are well, and having the same lovely weather that we are.
03.04.2025 07:11 — 👍 1 🔁 0 💬 0 📌 0
Today has been lovely, a grant was funded, had a couple of very productive and enjoyable meetings, got some stuff off my To Do list and the sun is shining. If a red squirrel came to the bird feeder at tea-time, that really would be the cherry on the top.
02.04.2025 15:48 — 👍 6 🔁 0 💬 1 📌 0


Many thanks to @vcornelius.bsky.social and @gsmaclennan.bsky.social for coming to Cork and sharing their wisdom on clinical trials today.
31.03.2025 17:35 — 👍 11 🔁 2 💬 2 📌 0

Audit and feedback: effects on professional practice - Ivers, N - 2025 | Cochrane Library
Select your preferred language for Cochrane reviews and other content. Sections without translation will be in English.
Interested in audit and feeback strategies and how they may improve healthcare practice? Then check out the updated Cochrane review on the topic, co-authored by our own Kristin Konnyu:
Audit and feedback: effects on professional practice - Ivers, N - 2025 www.cochranelibrary.com/cdsr/doi/10....
25.03.2025 15:51 — 👍 5 🔁 1 💬 0 📌 0
❗ Latest issue of @thelancet.bsky.social out today and featuring the TEMPO trial on the cover 🙂
My summary thread here: bsky.app/profile/gjho...
Open access paper here:
www.thelancet.com/journals/lan...
28.03.2025 14:28 — 👍 6 🔁 3 💬 0 📌 0
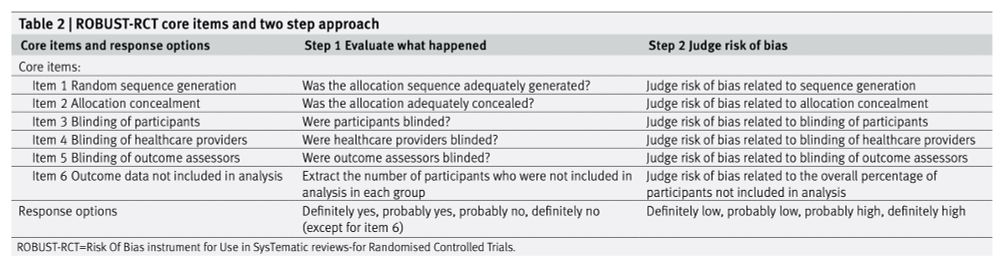
The 6 core items of the new risk of bias tool. Also in paper at the link.
Here's a new risk of bias tool for systematic reviews: ROBUST-RCT
www.bmj.com/content/388/...
Similar to an older Cochrane risk of bias tool, it looks like something that could actually be used, which is great. The team explicitly aimed to balance simplicity and methodological rigour.
27.03.2025 12:31 — 👍 2 🔁 3 💬 0 📌 0
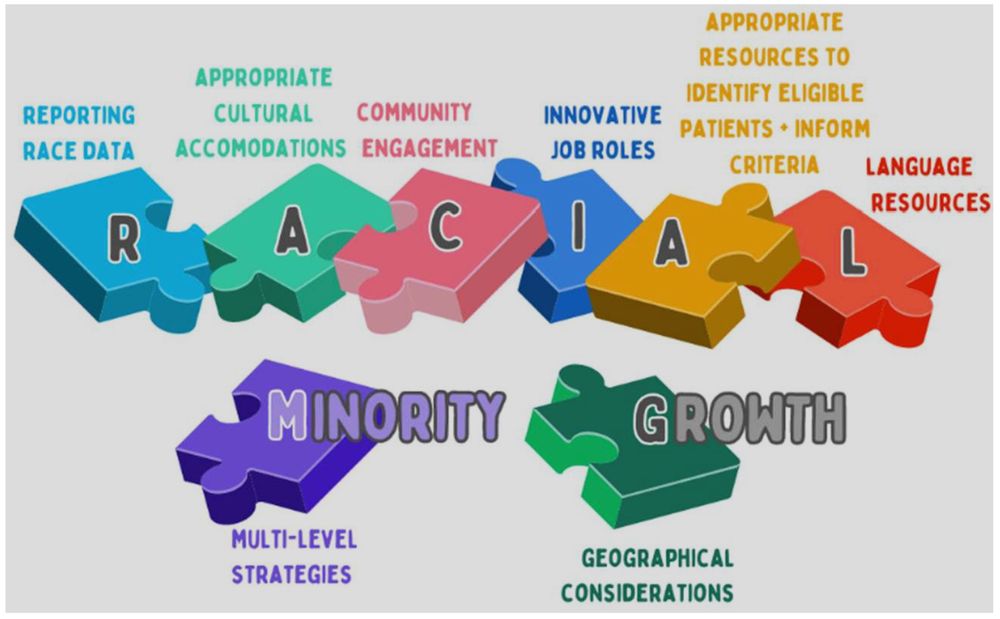
Figure 3 from the paper, which gives the eight themes identified in the review.
Here's an interesting rapid review on strategies to enhance the ethnic diversity of breast cancer clinical drug trials. It gives some trial recruitment and retention recommendations too.
www.academia.edu/2998-7741/1/...
27.03.2025 08:14 — 👍 3 🔁 2 💬 0 📌 0
Founder | Unwritten Health
Fighting for real health equity
https://unwritten-dispatches.beehiiv.com/
U-CARE is a research programme at Uppsala University aiming to increase mental health among patients and their significant others in relation to somatic disease.
https://www.uu.se/en/centre/u-care
A podcast about swallowing, swallowing disorders and the latest and greatest, or sometimes not so great, evidence!
Public health nutrition expert, researcher and advocate for nutrition, healthy lifestyles, and health policies to build a sustainable future.
ERASMUS+ Cooperation Partnerships funded grant to develop free training and education materials for trialists
Widening Access to Trials in Care Homes (WATCH) is a collaborative project led by Prof Roy Soiza at NHS Grampian. Aims to produce best practice guidance to enable the recruitment of care home residents into vaccine trials. Received Vaccine Innovation Fund.
Protecting and promoting the interests of patients and public in health and social care research. Making it easy to do research that people can trust.
Psychiatry Dr 🏴 | Research fellow @georgeinstitute @ImperialCollege | MESSAGE Co-PI www.messageproject.co.uk | www.katewomersley.com | #medsky #psychsky
The European Clinical Research Network Infrastructure's (ECRIN) mission is to support the conduct of multinational clinical research in Europe
The principal aim of Diverse PPI is to empower, educate & equip diverse ethnic communities so they can make informed decisions about their health & engage with research.
A research forum & platform representing diverse voices.
The NIHR ARC East Midlands is one of 15 ARCs (@nihrarcs.bsky.social) supporting applied health and care research, funded by the National Institute for Health and Care Research (@nihr.bsky.social).
Patient and Public Involvement Officer and Adviser. Clinical trials PPIE and inclusive research.
Experienced PPI lead in stroke research. Personal interest in mental health and neurodiversity research
Love music, visual arts, nature walking and gaming
Researcher for hire #AltAc.
On about games, books, cats, telly.
Always #LWithTheT
She/her
Metascience, statistics, psychology, philosophy of science. Eindhoven University of Technology, The Netherlands. Omnia probate. 🇪🇺
Medical Statistician at KU Leuven. My brain is like a snail but it gets there in the end (or not).
Professor in Clinical epidemiology, with a focus on medical tests and diagnostic test accuracy #SystematicReviews. Interested in #reproducibility of science. osiris4r.eu
#AcademicSky #EpiSky
linktr.ee/mattsydes
Head of Data-Driven Clinical Trials at NHS England.
Hon Prof of Clinical Trials & Methodology at UCL.
Hon Fellow at HDRUK.
Always keen to talk clinical trials & evidence-based healthcare.
Tied to kettle & news.
Local trips by bike.
Medical statistician and Director of PCTU, mum and reader of just about anything I can get my hands on (preferably whilst drinking tea and eating cake)
Scientist (PhD she/her), writer, cartoonist. 🦘 Blogging & Newsletter: Living With Evidence https://hildabastian.wordpress.com/ Mastodon enthusiast: @hildabast@mastodon.online
Director, Economic Statistics at ONS. Former Chief Economist at the Cabinet Office. All views my own.





















