To us, this was both unexpected and very interesting.
08.10.2025 13:44 — 👍 1 🔁 0 💬 0 📌 0To us, this was both unexpected and very interesting.
08.10.2025 13:44 — 👍 1 🔁 0 💬 0 📌 0In our case, we successfully obtained embryo-like structures; however, although the cells were reprogrammed to an earlier developmental stage, they did not appear to generate precursors. Instead, they transitioned directly into the various early embryonic fates.
08.10.2025 13:44 — 👍 1 🔁 0 💬 1 📌 0Thank you for the comment and question. We think that if we can reprogram cells into precursors of all embryonic lineages, they should then be able to self-organize into an embryo model.
08.10.2025 13:44 — 👍 1 🔁 0 💬 1 📌 0
Online Now! Signaling reprogramming via Stat3 activation unravels high-fidelity human post-implantation embryo modeling #stemcells
16.09.2025 19:09 — 👍 9 🔁 2 💬 0 📌 0
(8/8) Our v1.0, limitations remain (see Limitations section), but we hope, alongside other models, it can help advance human embryogenesis research.
Visit the lab website for free access to our study (under publications section)
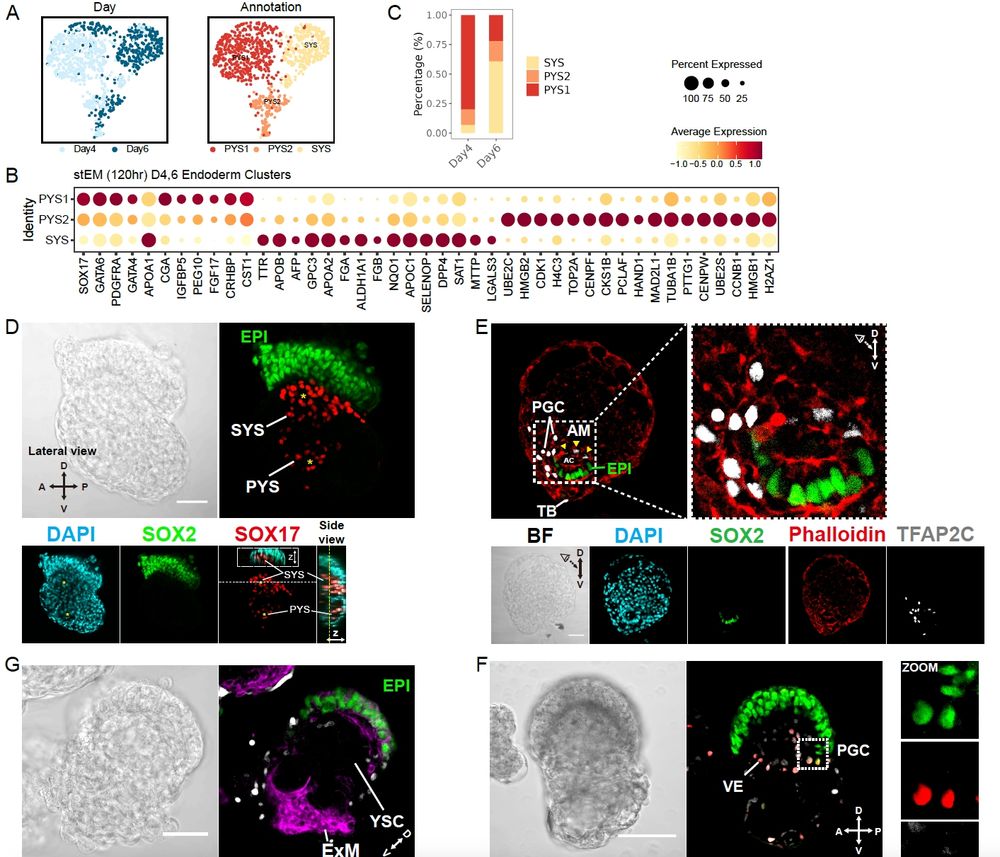



(7/8) There’s more inside: signaling landscapes, PGCs, primary-to-secondary yolk sac transition…
16.09.2025 16:11 — 👍 2 🔁 0 💬 1 📌 0
(6/8) The molecular signatures show strong alignment and high identity scores with human embryo references.
16.09.2025 16:11 — 👍 2 🔁 0 💬 1 📌 0
(5/8) ….And they keep going, entering gastrulation!
16.09.2025 16:11 — 👍 2 🔁 0 💬 1 📌 0
(4/8) If these cells can generate all early fates, then they should be able to do so in a self-organized manner, forming embryo-like structures……. and they did! (All we had to do was sit back and let the cells do their work.)
16.09.2025 16:11 — 👍 1 🔁 0 💬 1 📌 0
(3/8) The results went beyond expectations: alongside naïve epi, TE & hypoblast, we also saw extraembryonic mesoderm emerge 🤯
16.09.2025 16:11 — 👍 1 🔁 0 💬 1 📌 0
(2/8) From our work in the mouse system we hypothesised that STAT3 could reprogram human cells from a post- to a pre-implantation fate, but the breakthrough came only after we also added TGFβ inhibition.
16.09.2025 16:11 — 👍 1 🔁 0 💬 1 📌 0
(1/8) Very Happy to share our lab’s 3D post-implantation human embryo model, made via signalling reprograming!
🙌 Huge thanks to the entire team and our collaborators, special shout-out to @cc0447.bsky.social (led the work) and @jinjin-0101.bsky.social (comp. analysis).
www.cell.com/cell-stem-ce...

(3/3) Fast forward ⏩ @cc0447.bsky.social Jinyi Wu et al. investigated this in human cells. Curious to see what they pulled off? Stay tuned!
15.09.2025 01:51 — 👍 1 🔁 0 💬 0 📌 0
(2/3) … this was actually a side finding in that study🙃
www.cell.com/cell-stem-ce...
However, we thought this could be paralleling early embryo development and have since been exploring it to generate embryo models.
(1/3) A while back, my then-PhD student @htstuart.bsky.social stumbled on a finding that changed our lab’s direction 👀✨. Turns out, STAT3 mediated reprogramming generates intermediates (Gata6GFP+ cells) going through an earlier developmental stage with increased developmental potential 🚀
15.09.2025 01:51 — 👍 9 🔁 2 💬 1 📌 0I believe they will, but we’re not there yet.
09.09.2025 14:55 — 👍 0 🔁 0 💬 1 📌 0😀
09.09.2025 14:42 — 👍 1 🔁 0 💬 0 📌 0
💓 Researchers in the @josesilvalab.bsky.social used #SmallMolecules to convert mouse ESCs into embryo founder cells, which generated a complete embryo model!
Article: bit.ly/4orpbLb
Episode with discussion: bit.ly/4oTURt2
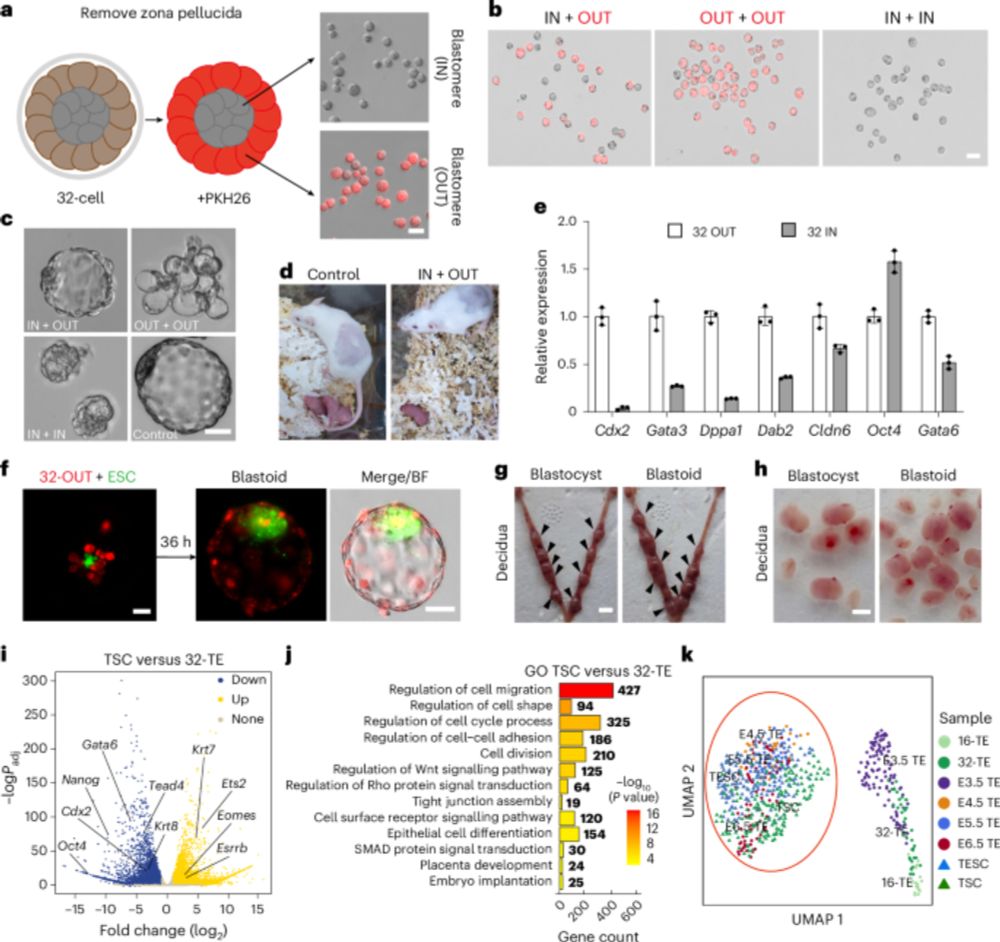
🥂Congrats to Gao, Li & co for their new @natcellbio.nature.com study: they derive #trophectoderm stem cells from 32-cell mouse #embryos. These cells represent an early trophectoderm state, develop into #placenta cells and organoids and contribute to blastoid generation.
rdcu.be/eAKzp
bit.ly/4fyZ9Sg
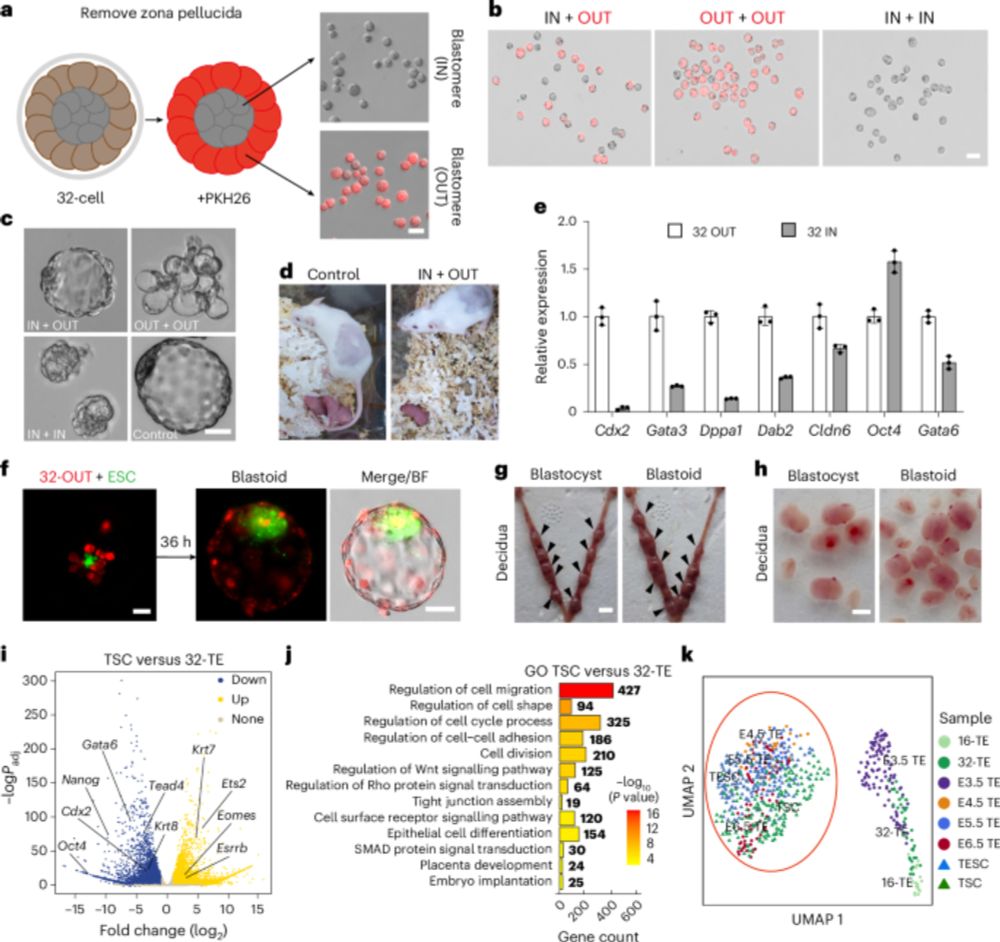
Capturing early TE stem cells. From Zhang Jian’s lab, “Mouse trophectoderm stem cells generated
with morula signalling inducers capture an early trophectoderm state”, with a small contribution from us.
www.nature.com/articles/s41...
Thank you so much Alex!
07.08.2025 20:56 — 👍 0 🔁 0 💬 0 📌 0
A big thanks to all authors, especially Professor Naihe Jing and Penglei Shen for guiding our embryo model into organogenesis, and to our PhD students Jiahui Huang and Wei Guan for their efforts. And of course, to Huanhuan Li, the driving force behind this work.( 3/3)
07.08.2025 15:58 — 👍 2 🔁 1 💬 1 📌 0
Starting from PSCs, a brief reprogramming step gives rise to “embryo founder-like cells” (EFCs). EFCs segregate embryonic and extraembryonic lineages and self-organize to closely mirror natural embryo development, both morphologically and at the molecular level.
authors.elsevier.com/sd/article/S...
Very happy to share the work of Huanhuan Li et al. While not a natural embryo, you might not have known that if I hadn’t told you. (1/3)
07.08.2025 15:58 — 👍 14 🔁 8 💬 3 📌 0
Join the VGZT Eastern KEYNOTE lecture ☀️☕ TOMORROW
🗓️ Wednesday July 09
⏰ 13:30 IST / 17:00 JST / 8:00 UTC / 10:00 CET
Featuring:
1) Maithreyi Narasimha
👉Positioning tissues during Drosophila morphogenesis
Jose Silva @josesilvalab.bsky.social
👉From founder cells to organogenesis-stage embryo model
Thank you VGZT community for your active participation 🌎
See below the session flyers for the rest of the season:
🌞 Eastern sessions: Happening on Wednesdays.
Our keynote for this season: Maithreyi Narasimha and @josesilvalab.bsky.social
[1/2]
Very grateful to the @lundstem.bsky.social and Filipe Pereira for hosting me. I truly enjoyed meeting and discussing with many colleagues at the Center. I also received very interesting feedback that will hopefully lead to collaborations and help further improve our embryo models.
21.02.2025 17:32 — 👍 3 🔁 0 💬 0 📌 0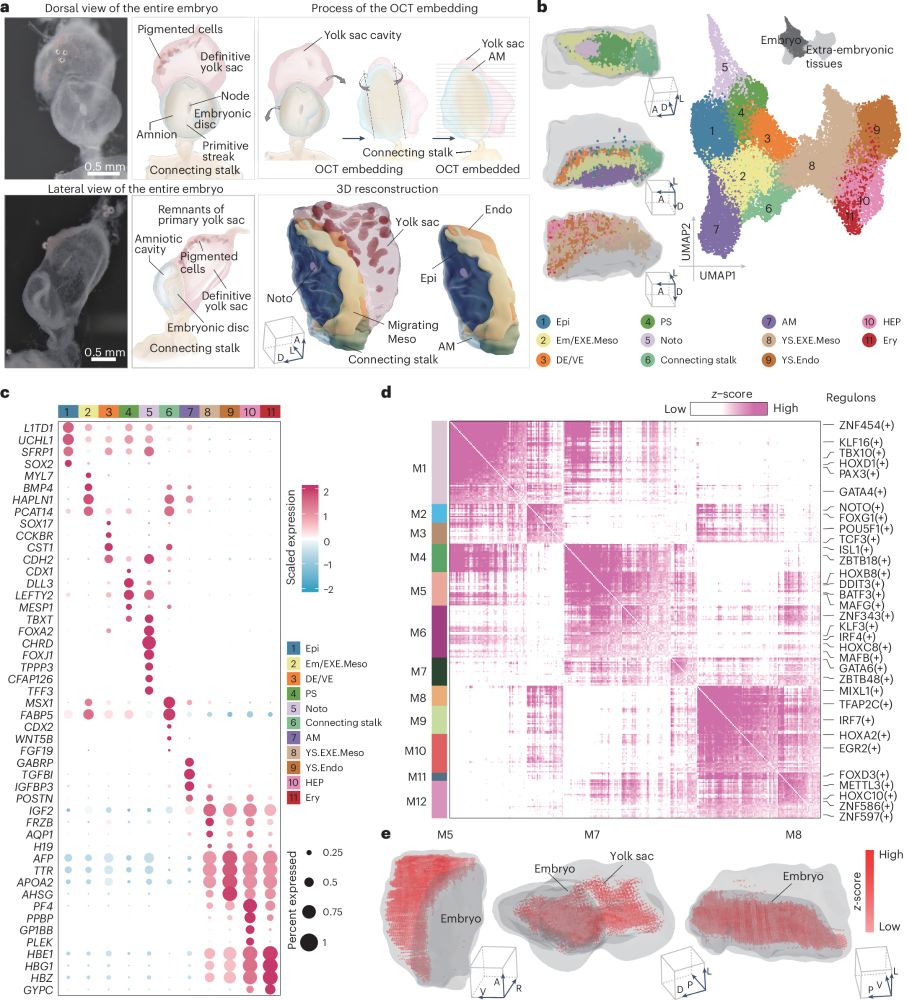
An interesting study characterising human embryo gastrulation!
rdcu.be/d5VmN
Many congratulations Amanda!!!!
03.12.2024 14:17 — 👍 1 🔁 0 💬 0 📌 0