It's possible to apply for the Hubrecht Talent Program again!
The HTP aims to promote scientific excellence in the Netherlands by supporting talented minority students in pursuing a career in scientific research.
Read more in the flyer and on www.hubrecht.eu/about-us/hub...
28.04.2025 09:28 — 👍 5 🔁 5 💬 0 📌 1
How do embryos ensure precise tissue patterning? It’s all about timing cell divisions! Our new preprint reveals how cell proliferation syncs with signaling oscillations to regulate precision of somite formation and growth. Check the full story: www.biorxiv.org/content/10.1...
11.03.2025 12:29 — 👍 68 🔁 16 💬 2 📌 1
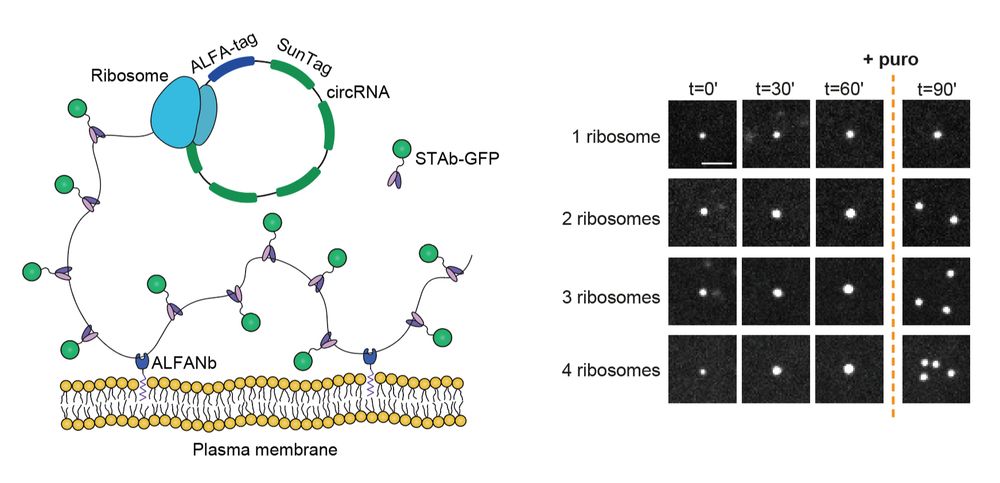
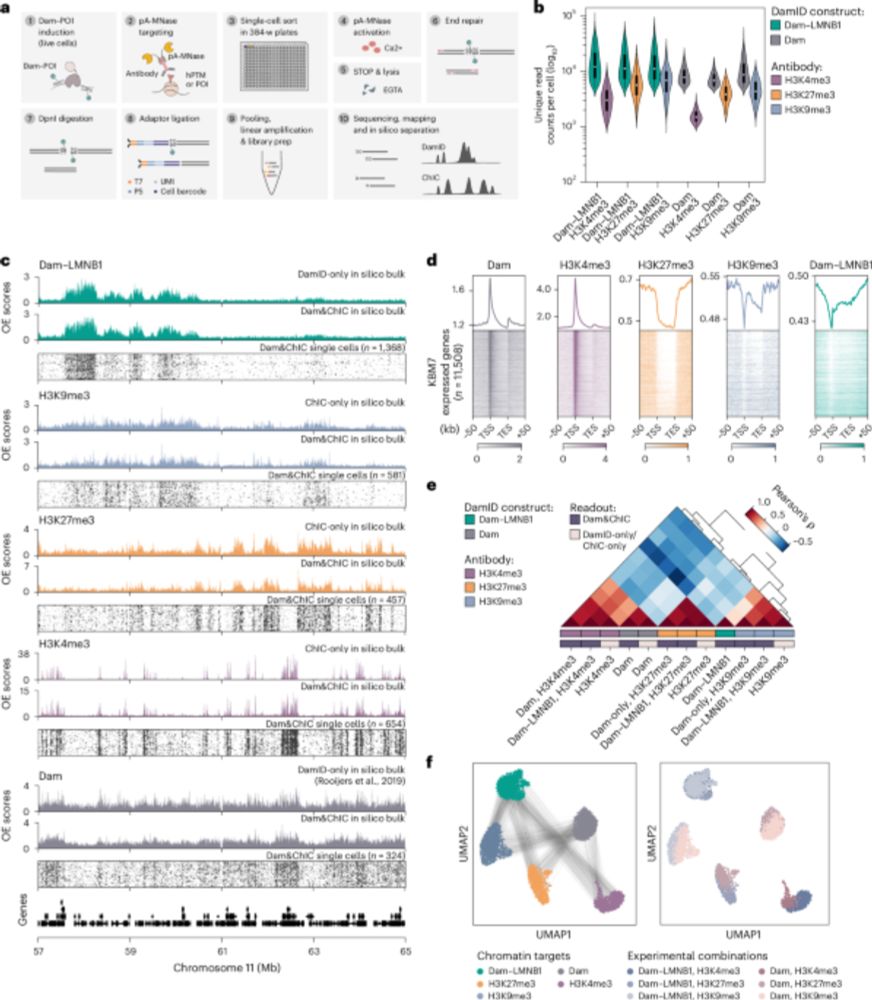
Our paper is out! We delevoped a method to follow individual translating ribosomes for hours in living cells, and discovered that ribosomes are great friends and help each other in problematic situations:
www.cell.com/cell/fulltex...
03.02.2025 08:31 — 👍 43 🔁 11 💬 0 📌 0
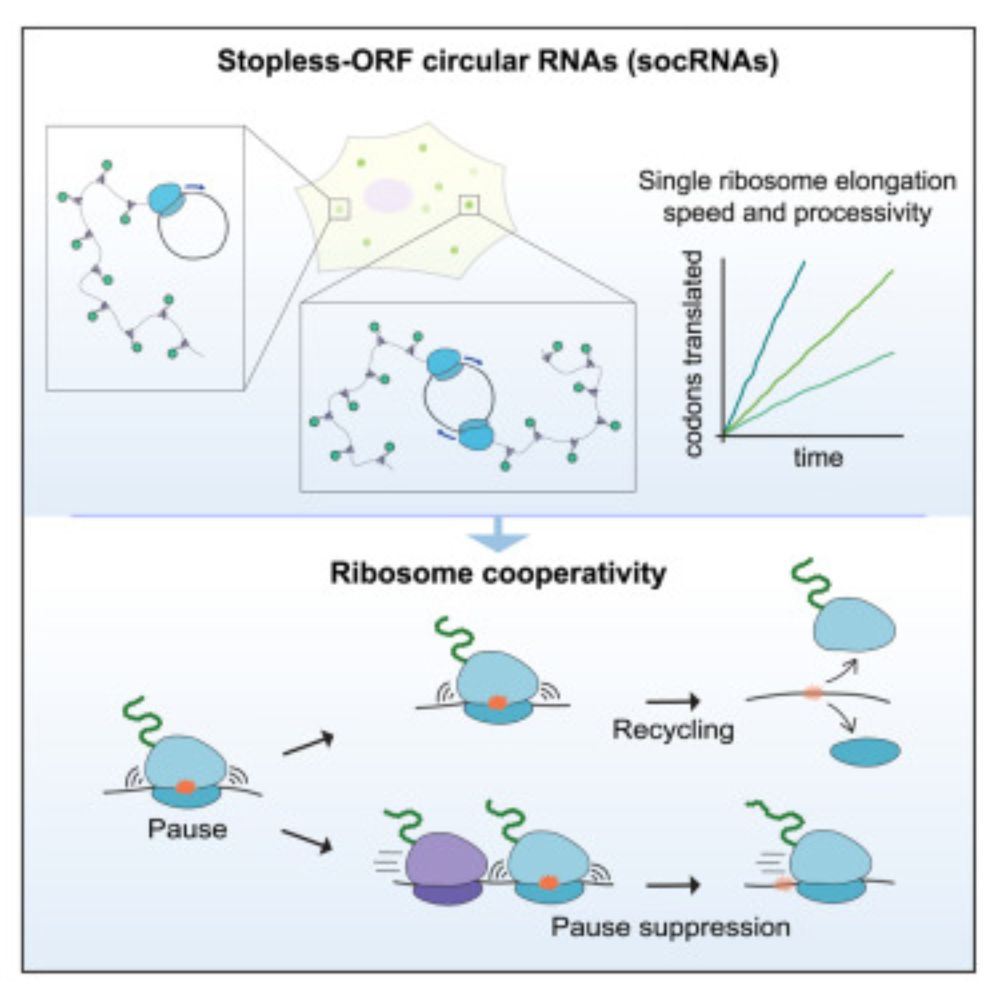
Long-term imaging of individual ribosomes reveals ribosome cooperativity in mRNA translation
Ribosomes cooperate through transient collisions to ensure efficient translation.
Our paper on Stopless-ORF Circular RNAs (socRNAs) is now out in Cell. By high-res tracking and comparing translation by either single or multiple ribosomes, we find that ribosomes cooperate to overcome pausing to ensure fast and efficient translation
www.cell.com/cell/fulltex...
03.02.2025 07:38 — 👍 113 🔁 46 💬 4 📌 6
This was a big team effort by the bsky-less @Huib, @janinschoko.bsky.social, @baarsmatthijs.bsky.social and Jakob in @marvintanenbaum.bsky.social lab. Also, a great collaboration with @RonFouchier and @antonelladost.bsky.social & @hansclevers.bsky.social on the patient samples and airway organoids!
21.01.2025 10:16 — 👍 2 🔁 1 💬 0 📌 0
We envision that the tools described in this manuscript open up new avenues to study IAV biology in unprecedented detail. Conceptually, with virus-specific modifications our imaging systems are even broadly applicable to many different (-)RNA viruses.
21.01.2025 10:16 — 👍 0 🔁 0 💬 1 📌 0
(3) Finally, we found that even when vRNPs are present, they often lack transcriptional activity. As a result, most infected cells only transcribe very few vRNPs. We conclude that viral transcription itself is a highly limiting factor in determining the successful outcome of IAV infections.
21.01.2025 10:16 — 👍 0 🔁 0 💬 1 📌 0
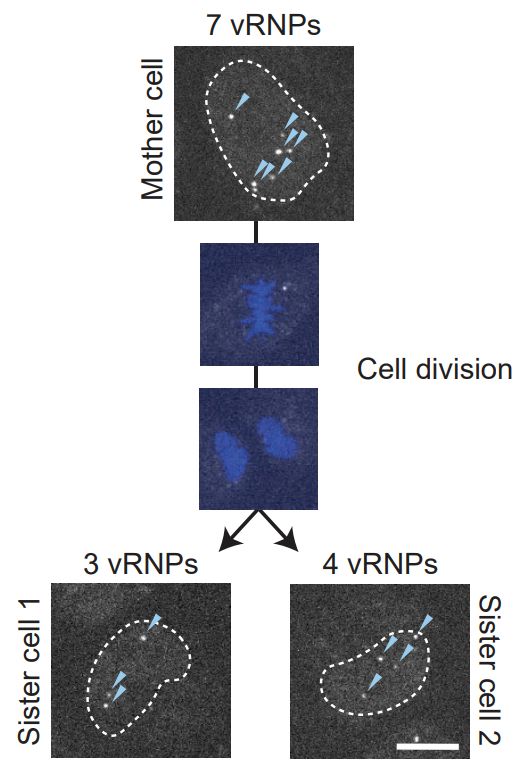
(2) Mitosis causes vRNPs to be distributed over two separate sister cells. Therefore, these sister-cells often end up having incomplete sets of genome segments. We termed this “viral aneuploidy”, akin to chromosome segregation errors occurring during host cell division.
21.01.2025 10:16 — 👍 0 🔁 0 💬 1 📌 0
(1) As previously reported, due to the segmented nature of the IAV genome, virions can lack one or multiple genome segments. We confirmed this both by using live-cell imaging of incoming vRNPs and by performing smFISH on viral particles.
21.01.2025 10:16 — 👍 0 🔁 0 💬 1 📌 0
Finally, we wondered what the underlying reason for the observed defects in viral gene expression could be and found three mechanisms:
21.01.2025 10:16 — 👍 0 🔁 0 💬 1 📌 0
We analyzed naturally occurring single-gene KOs (cells expressing all but one viral gene) - which are otherwise hard to generate because most viral genes are essential - to study viral protein function and gained insights into which proteins are important for viral replication and nuclear export.
21.01.2025 10:16 — 👍 0 🔁 0 💬 1 📌 0
We wondered why so many infections fail to progress through all life-cycle stages. We combined our live-cell imaging technologies with multiplexed smFISH and found that many viruses fail to transcribe one or multiple genes.
21.01.2025 10:16 — 👍 4 🔁 1 💬 1 📌 0
Using single-cell traces of many hundreds of cells, we constructed a kinetic map of IAV infections, revealing large heterogeneity in the timing and success rates of the individual steps in the viral life cycle. Infections are very unsuccessful, with only ~4% of them resulting in progeny production.
21.01.2025 10:16 — 👍 0 🔁 0 💬 1 📌 0
To capture the late-stage event of new virions budding off, we developed a second, orthogonal technique that visualizes the build-up of HA-protein on the cell surface of infected cells and even labels budding virions.
21.01.2025 10:16 — 👍 1 🔁 0 💬 1 📌 0
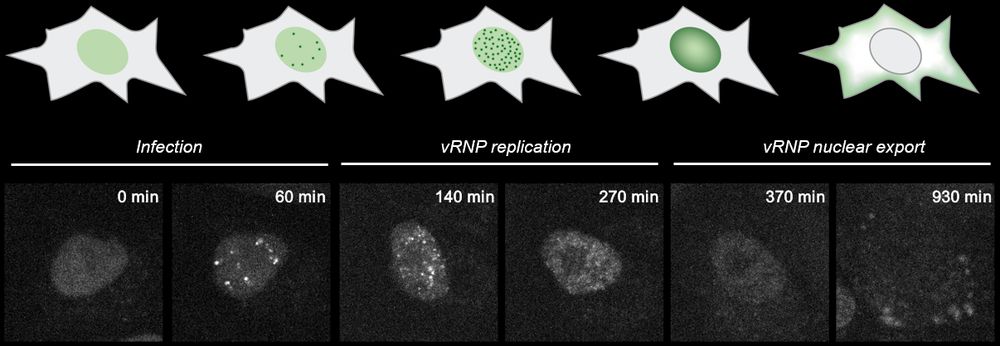
Further we can follow IAV over time and observed vRNPs replicating and later getting exported from the nucleus to allow assembly of new virions.
21.01.2025 10:16 — 👍 1 🔁 0 💬 1 📌 0
Using this technology we show for the first time the moment that virions fuse with the endosomal membrane and release vRNPs step-by-step into the host cell
21.01.2025 10:16 — 👍 2 🔁 0 💬 1 📌 0
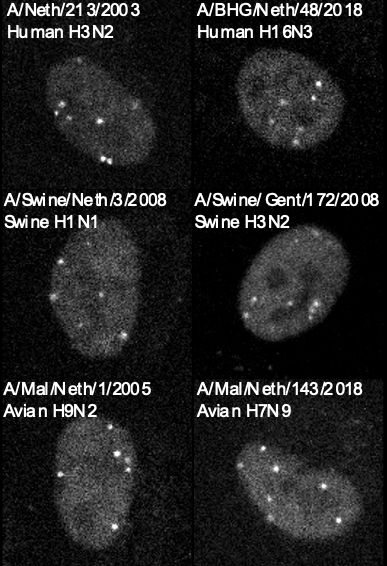
The NP nanobody recognizes a broad range of IAV strains, including swine, avian, and human strains, and even viruses directly isolated from patient samples! Since there is no need to genetically modify the viruses, it is very easy to do experiments with new strains and isolates.
21.01.2025 10:16 — 👍 0 🔁 0 💬 1 📌 0
Influenza viruses are segmented, negative-sense RNA viruses that, like other (-)RNA viruses, have their genomes encapsidated by nucleoproteins (NP). We exploited this characteristic to visualize single, unmodified IAV genomes by expressing a fluorescently labelled nanobody that binds to NP.
21.01.2025 10:16 — 👍 0 🔁 0 💬 1 📌 0
Very happy to share our preprint on visualizing the life cycle of Influenza viruses using single-molecule imaging! 🥳 We developed two techniques to visualize infections of unmodified influenza viruses in live cells from endosomal release to budding of new viruses. For more details&videos see below ⬇️
21.01.2025 10:16 — 👍 64 🔁 14 💬 2 📌 0

I am really happy to announce the first Hubrecht Symposium on March 13th 2025! We will organize these yearly events on a specific topic in molecular and developmental biology to emphasize the importance of fundamental research for Dutch science. Free of charge! www.hubrecht.eu/hubrecht-sym...
29.11.2024 12:30 — 👍 44 🔁 18 💬 0 📌 2
Congrats! 🥳
20.11.2024 06:43 — 👍 1 🔁 0 💬 1 📌 0
senior scientist at Janelia | single particle tracking & #microscopy 🔬 | PhD on single-molecule enzymatic dynamics | my path: 🇩🇪→🇺🇸→🇸🇪→🇺🇸 | https://scholar.google.com/citations?user=UzGfR3MAAAAJ&hl=en
Industry Scientist | Sequencing and Cells
🦠 Postdoc @jcvi.org
🧠 PhD @utah.edu
Postdoc at the Hubrecht Institute 🧬🩸
DNA replication stress, BMF and blood aging
A platform for life sciences. Publications, research protocols, news, events, jobs and more. Sign up at https://www.lifescience.net.
SNSF Postdoctoral Fellow | Pasca Lab at Stanford neurodegeneration and development, organoids, high-throughput screening
An international online community and seminar series about chromatin and gene regulation.
Join the community on Discord: http://discord.gg/dXqT89r
Subscribe to our mailing list: https://stats.sender.net/forms/bmVp3d/view
Professor and Head of @WUSM_BMB. Studying protein PTMs in chromatin biology. #TeamMassSpec cheerleader. Working towards changing academia. Views my own 🇺🇸 🇲🇽
Interested in how cells use genes to create shape, form and function in #embryos as well as in Societal Wellbeing
#NotInTheGenes #InNumbersWeTrust #gastruloids #gastrulation
Group leader at the Danish Cancer Institute and Professor at University of Copenhagen #NNFCPR. Interested in epigenetics, genome maintenance, aging & cancer, creativity & innovation, society in general. Opinions are my own.
Enhancers, 3D genome organisation, pluripotent stem cells
Babraham Institute and Enhanc3D Genomics
Decoding how the gut thinks 🦠🪱🧠💪
Neuroscientist with interests in
#EnergyMetabolism #EntericNeurons #Fats
@crick.ac.uk @institutducerveau.bsky.social
Associate Professor, University of Pennsylvania, Penn Epigenetics Institute
3D Genome structure and function
https://ericjoycelab.com/
PhD student at Galli lab, Hubrecht Institute. Developmental genetics, cell-cycle regulation and C. elegans ~~~
Wandering around the complexities of genome regulation and anything connected to it 🧬
A dedicated fan of smiling out loud!
Currently at Sorbonne Université
The European Molecular Biology Laboratory drives visionary basic research and technology development in the life sciences. www.embl.org
🧬 Join our world-class courses, conferences, and workshops at the forefront of molecular life science and its applications. 👩🏻🔬 www.embl.org/events/ 🔬
We are a research lab at the Netherlands Cancer Institute. We develop and apply new genomics tools to study genome biology and gene regulation.
#Chromatin biologist/biophysicist @NIG & SOKENDAI in Japan.
Chromatin is very dynamic and flexible, but NOT regular!!! 🧪🧬🔬
My career and work: http://bit.ly/2CuF4L5
Lab HP: https://bit.ly/3F1a8nk
YouTube Seminar: https://bit.ly/4fYZiOj
PhD student @EMBL in the Krebs Lab