Update: 在最近公布的《全国药品集中采购文件》中,增加了两项关于集采药品生产环节变更的规定:变更时需公开变更内容、未发生变更的企业在同等价位时优先入选。
这两项规定明显弱于之前征求意见稿中的硬性规定:首个中选周期内不得进行重要生产环节的变更,否则取消中选资格。
大概是多方博弈的结果,baby steps也好吧。
22.09.2025 17:14 — 👍 4 🔁 1 💬 0 📌 0
You have a great point! — re if most grants are to universities then that’s the hidden variable. The majority of grants/contracts are not to universities, and I see similar results when excluding universities from the analysis.
27.03.2025 20:45 — 👍 3 🔁 0 💬 1 📌 0
Relevant analysis: bsky.app/profile/airm...
23.03.2025 13:40 — 👍 3 🔁 2 💬 0 📌 0
It is therefore possible that they made cancellations unbiasedly across the Trump-Harris political spectrum but preferentially disclosed ones to Harris counties for publicity purposes.
23.03.2025 13:38 — 👍 93 🔁 7 💬 3 📌 0
Potential caveat: DOGE doesn't specify how it chose certain contract/grant cancellations to disclose. They claim the ones disclosed represent "~30% of total savings".
23.03.2025 13:38 — 👍 82 🔁 7 💬 1 📌 0
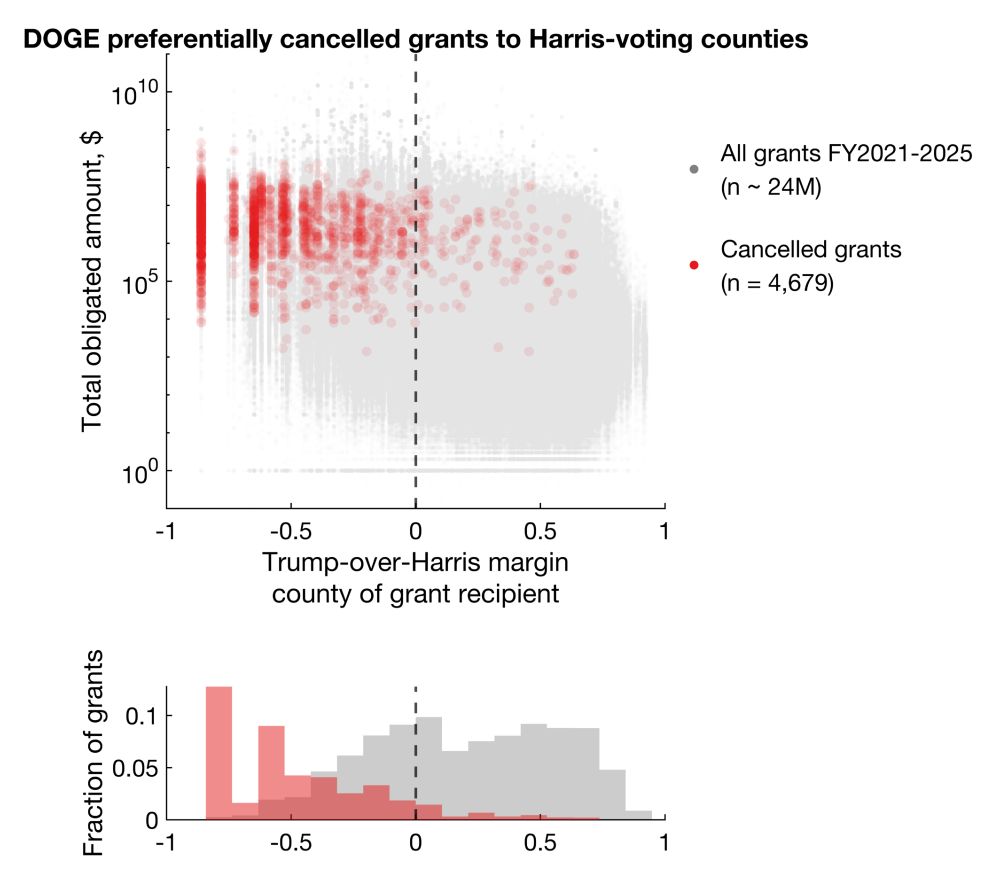
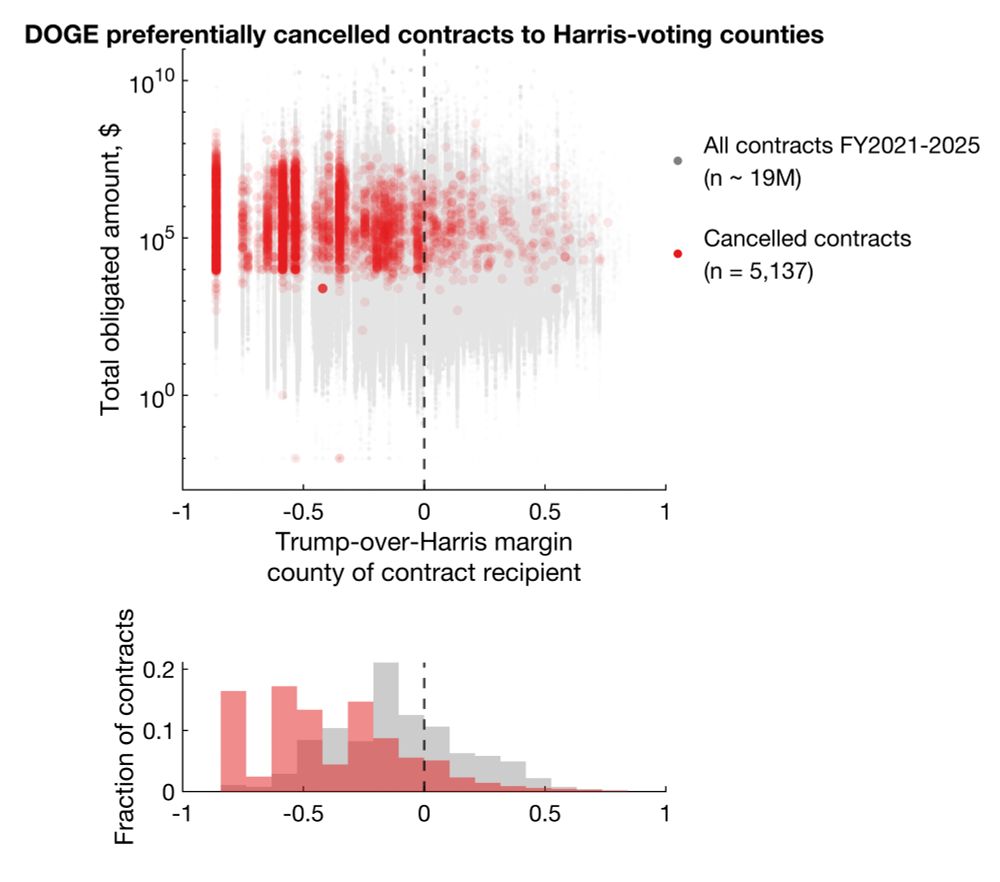
Clearly, the background/control sets are distributed across the Trump-Harris spectrum, but the cancellations are biased towards Harris counties.
Statistically significant differences shown with Mann-Whitney and Kolmogorov-Smirnov tests (p < 1e-100).
Large cluster on very left is DC.
23.03.2025 13:38 — 👍 81 🔁 15 💬 1 📌 0
To answer this, I need a good background/control set. I compiled all contracts/grants from FY2021-2025 on USAspending, totaling ~19M/24M. ~99% of all cancelled contracts/grants were from this period.
Similar results were seen with more restricted time periods, e.g. only FY2024.
23.03.2025 13:38 — 👍 74 🔁 5 💬 1 📌 2
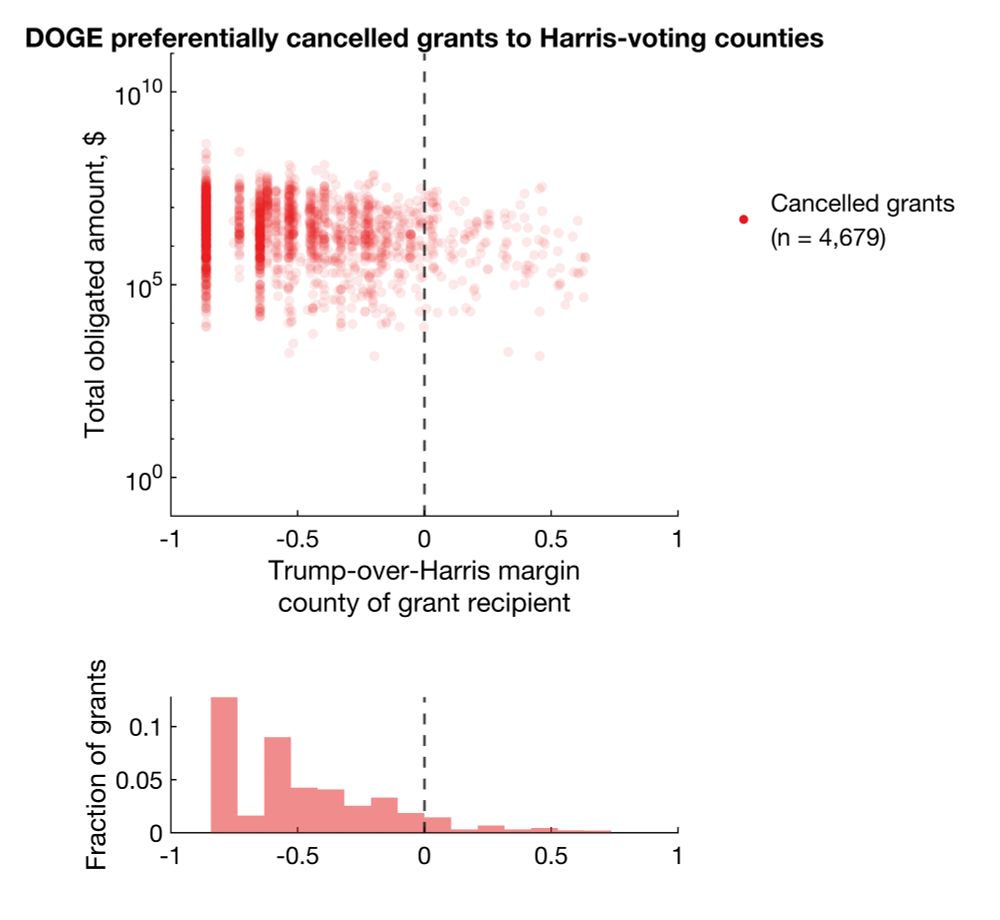
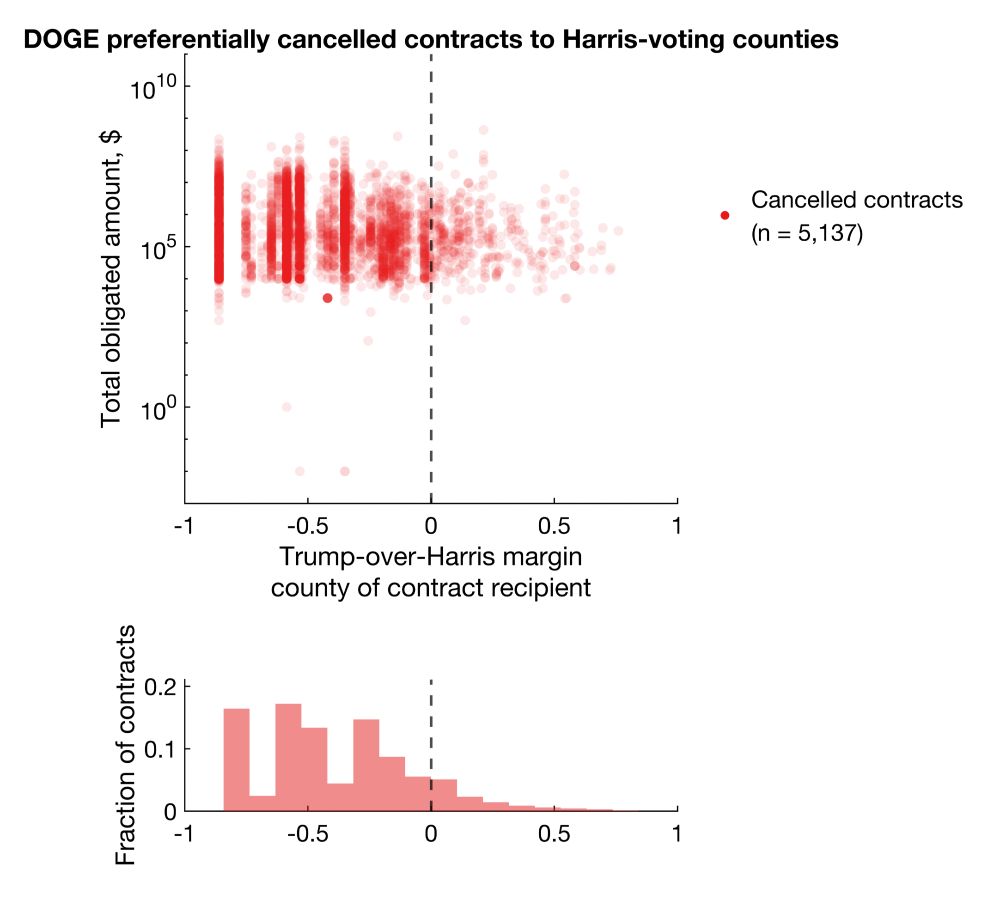
I plotted every cancellation, with total dollar amount obligated on the y axis against Trump-over-Harris margin on x.
Clearly, there's a bias for more cancellations in Harris counties. But does this reflect true bias or simply more contracts/grants awarded to Harris counties?
23.03.2025 13:38 — 👍 94 🔁 11 💬 1 📌 0
I used election data scraped from Fox News (www.foxnews.com/elections/20...) by github.com/tonmcg/US_Co...
For each contract/grant, I found Trump's popular vote margin over Harris in the recipient county.
Similar results were seen with NYT's election data (github.com/nytimes/pres...).
23.03.2025 13:38 — 👍 74 🔁 5 💬 1 📌 0
These metadata include total dollar amounts obligated, dates, and information on contract/grant recipients (address, county, congressional district, etc).
I extracted county info (FIPS code) and cross-referenced them to county-level presidential election data from 2024.
23.03.2025 13:38 — 👍 84 🔁 6 💬 2 📌 0
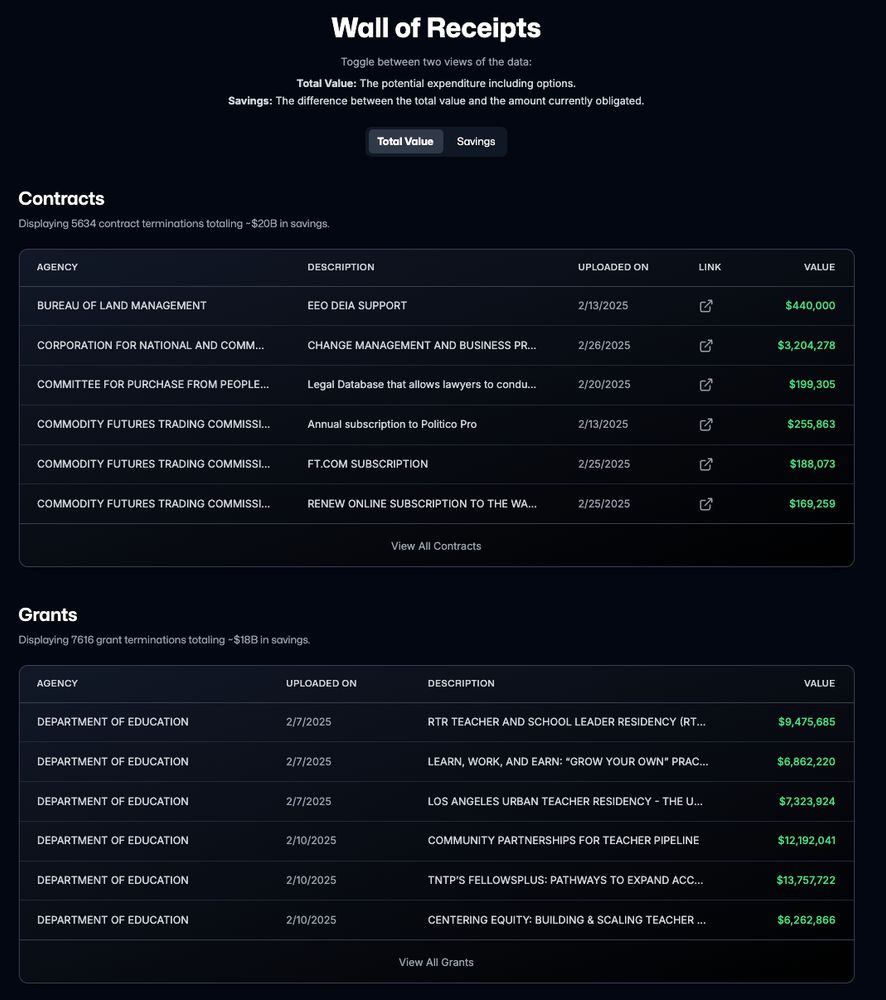
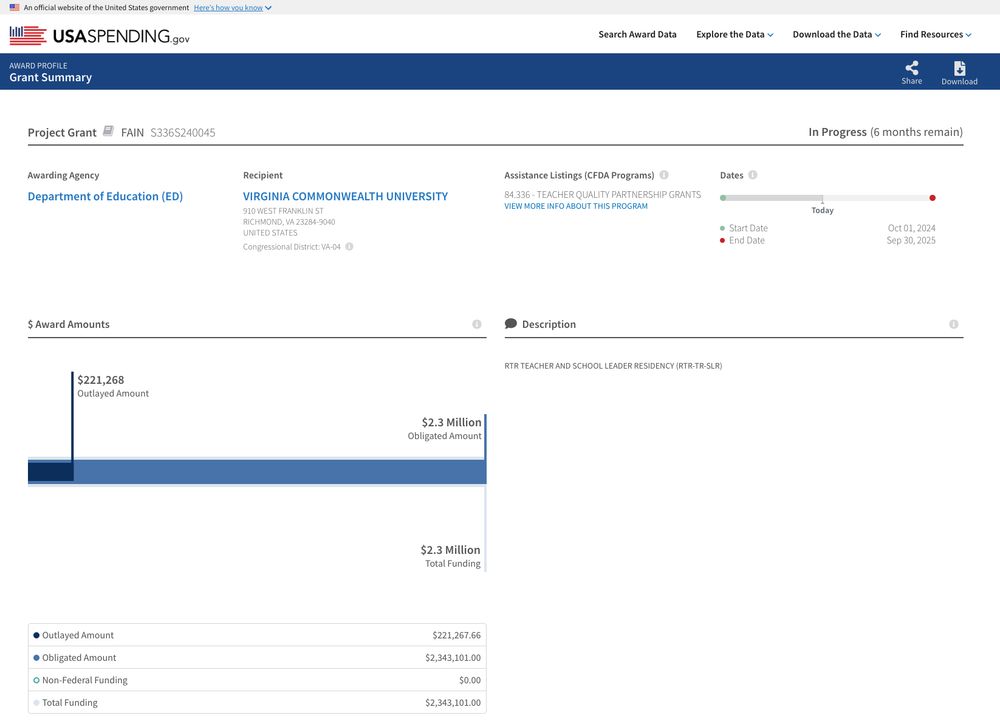
I retrieved all publicly available cancellations from DOGE on 3/22, which according to DOGE is a subset of all cancellations.
I then cross-referenced them to official spending data on USAspending using links provided by DOGE and ended up with 5,137 and 4,679 contracts and grants with rich metadata.
23.03.2025 13:38 — 👍 127 🔁 17 💬 2 📌 0
Data source:
doge.gov/savings — cancelled federal grants and contracts
USAspending.gov — contract/grant recipient info
github.com/tonmcg/US_Co... & github.com/nytimes/pres... — county-level election data
23.03.2025 13:38 — 👍 130 🔁 21 💬 2 📌 0
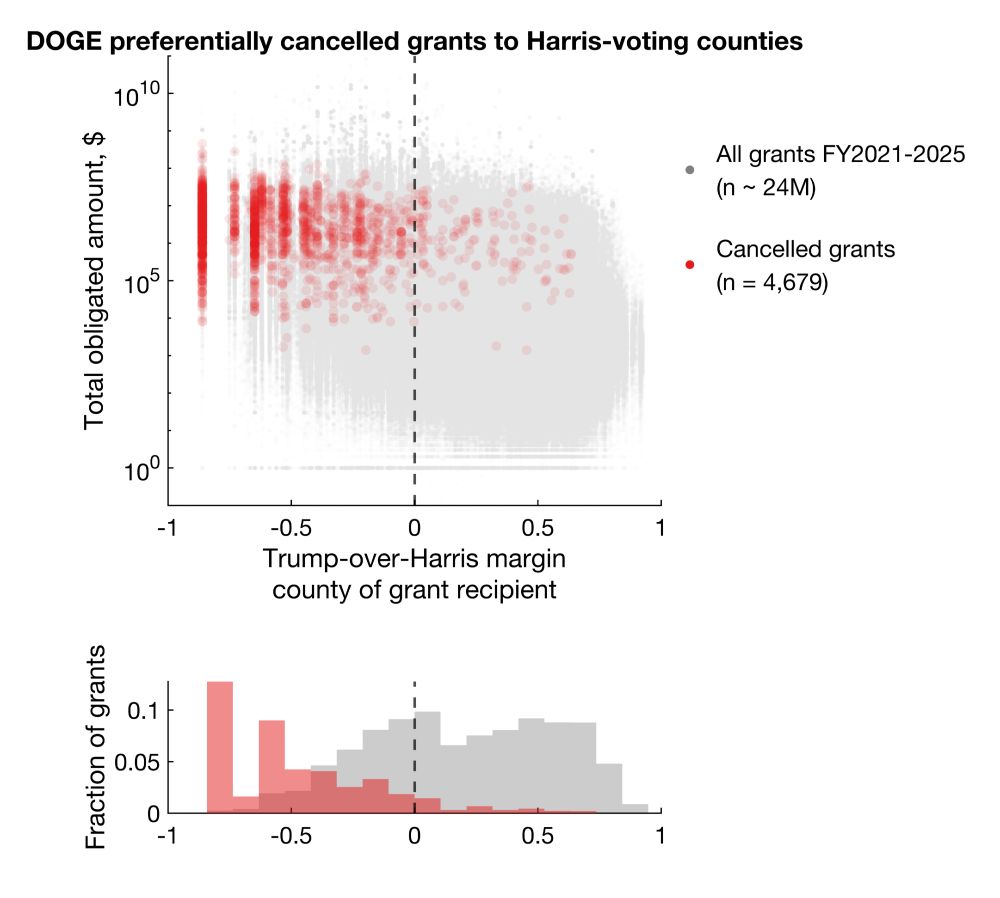
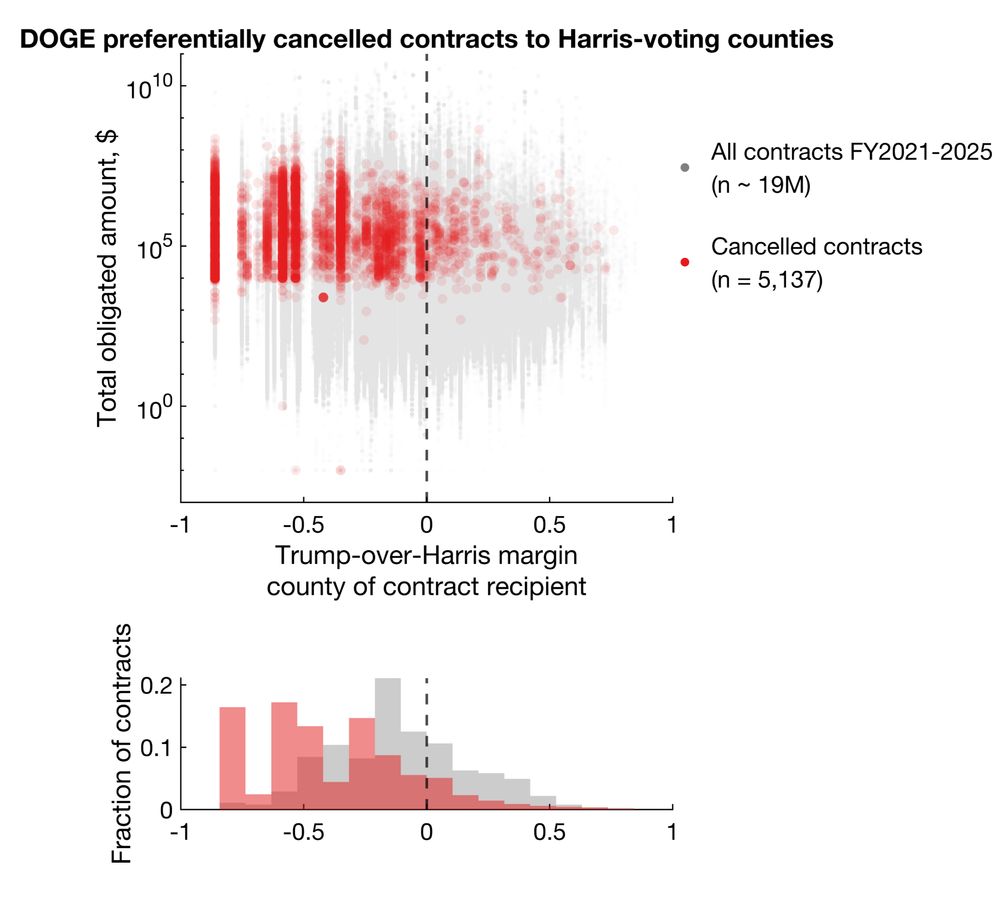
DOGE/Musk preferentially cancelled grants and contracts to recipients in counties that voted for Harris in 2024.
Among cancellations with election data available, 92.9% and 86.1% cancelled grants and contracts went to Harris counties, representing 96.6% and 92.4% of total dollar amounts.
23.03.2025 13:38 — 👍 2230 🔁 1106 💬 72 📌 145
Again, I’m in no way against centralized volume-based procurement and generics. In fact, I think they’re fundamentally a great idea for patients, given that their safety and effectiveness are demonstrated with rigorous testing and proper regulatory oversight.
18.02.2025 18:40 — 👍 2 🔁 0 💬 0 📌 0
While these production-related changes do not necessarily affect drug efficacy or safety, NMPA does not disclose the regulatory tests and inspections done (if any) that addresses whether new suppliers, processes, or sites materially impact drug composition or performance.
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
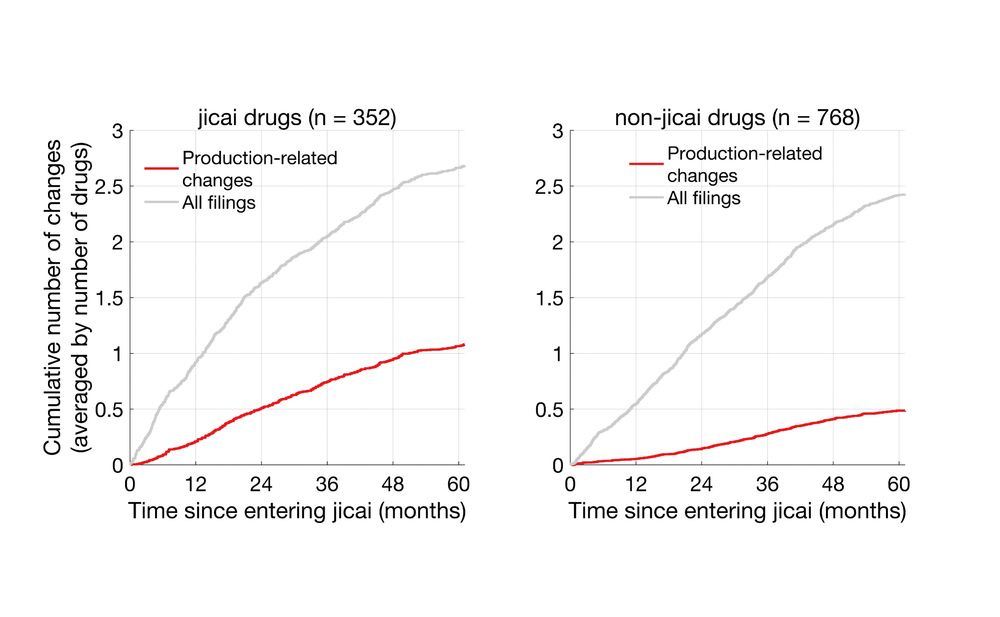
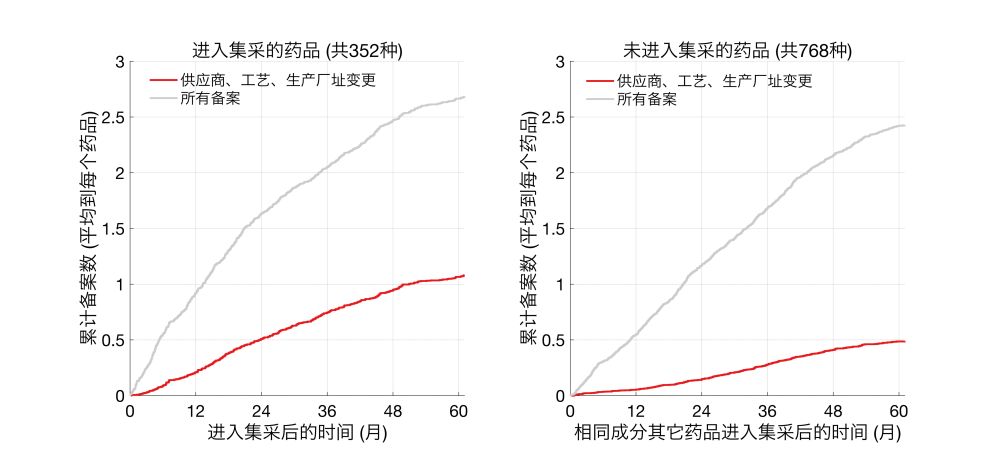
Expanding this to the entire dataset, I found 121 drugs that had both jicai and non-jicai generics, n = 352 and 768 respectively.
While the number of total filings were similar between the two groups, production-related changes in jicai drugs were ~2-fold that of non-jicai.
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
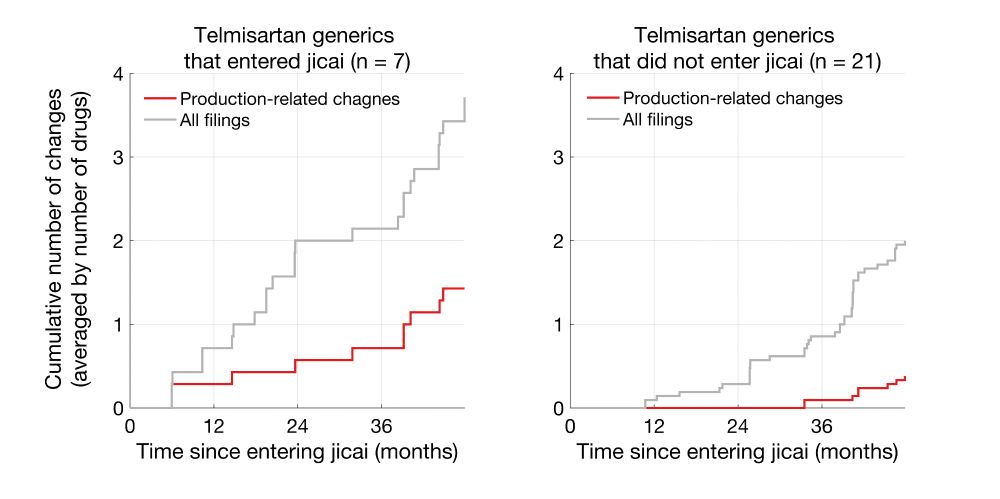
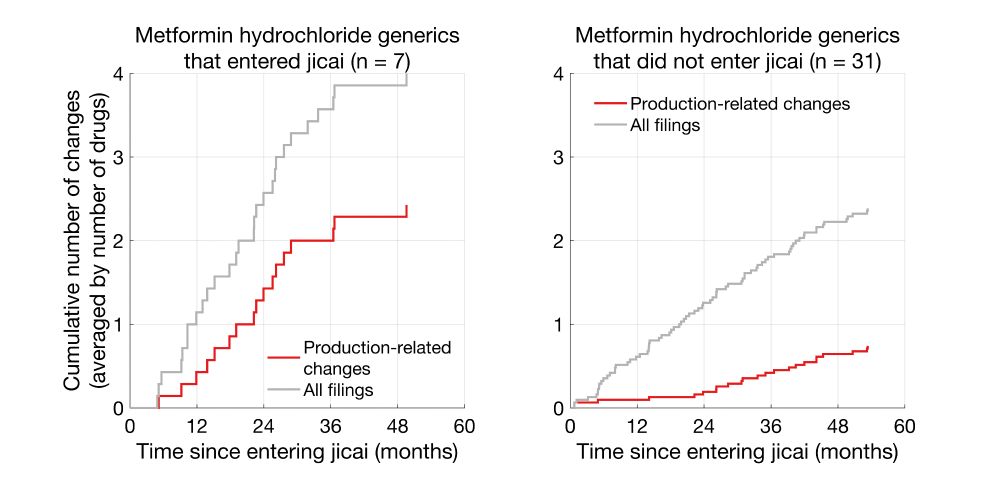
Here I am plotting the cumulative number of changes, averaged by the number of drugs.
Clearly, Telmisartan generics that entered jicai underwent more production-related changes than non-jicai generics.
Same trends were also seen for metformin hydrochloride generics.
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
For example, there are a total of 28 Telmisartan generics that passed BE, and 7 of these entered jicai on 2021/2/8. I tabulated the number of production-related changes (supplier, process, or site) that happened for jicai vs non-jicai drugs starting on 2021/2/8.
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
To test this more rigorously, I compared jicai drugs with drugs that (1) share the same active ingredient and (2) passed BE tests, but (3) did not enter jicai.
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
An interesting finding here is that percentages of drugs that underwent postapproval changes are higher for jicai drugs than generics. A hypothesis is that jicai drugs undergo more postapproval changes due to cost pressures associated with low bids.
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
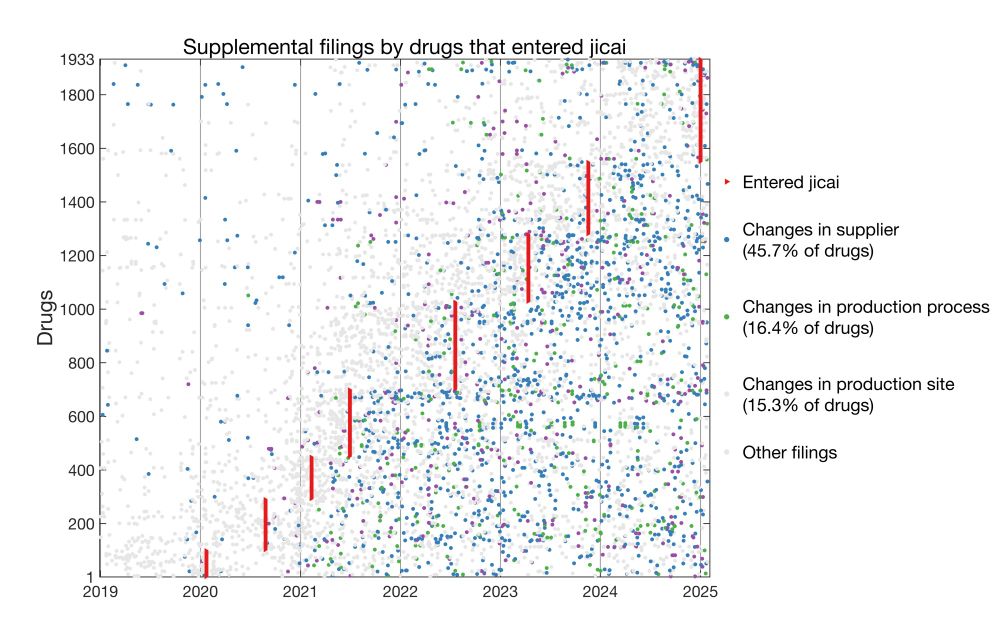
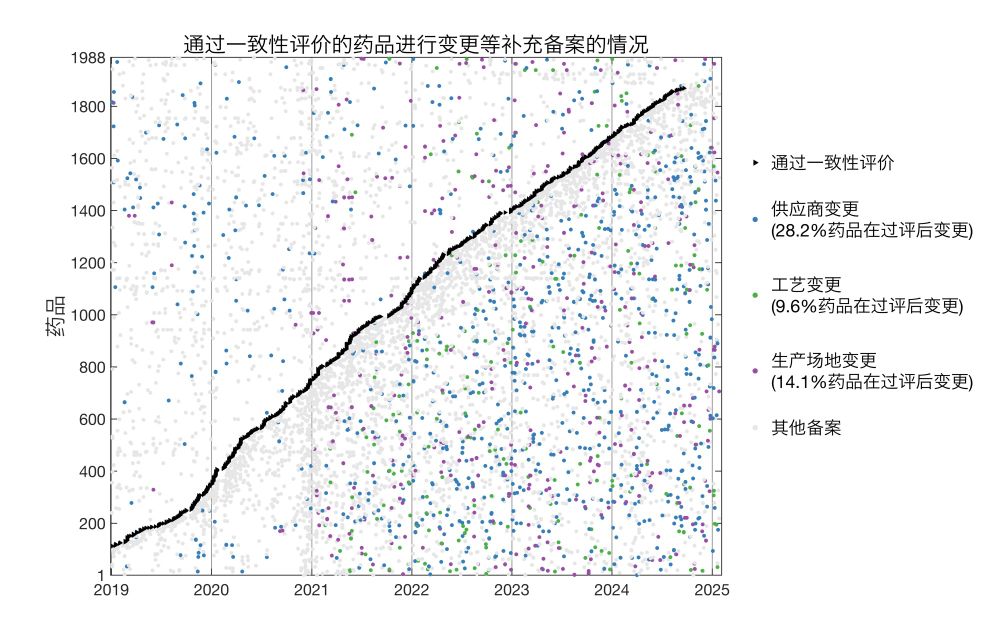
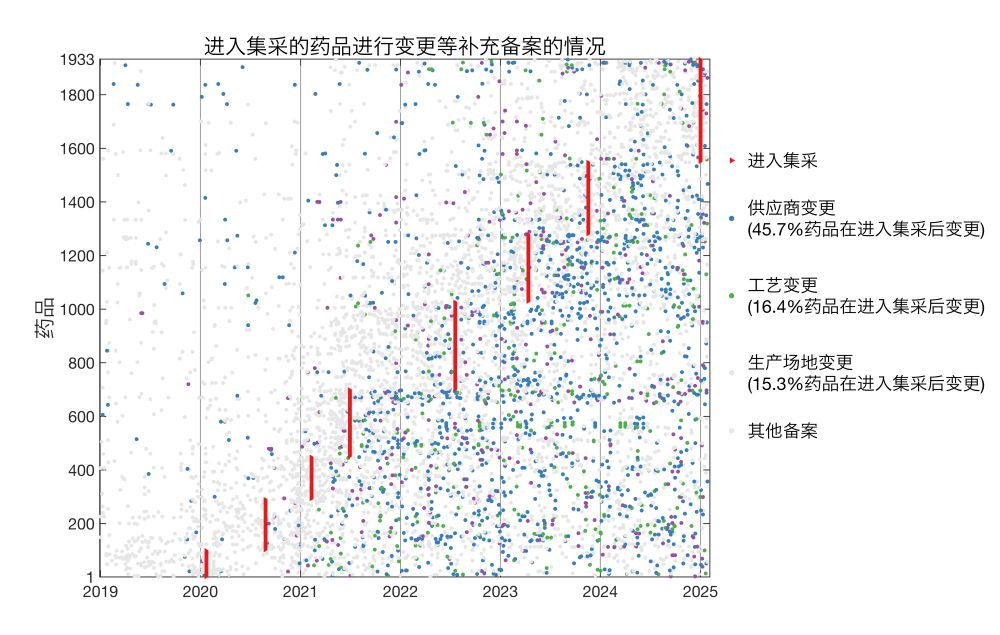
Drugs that entered jicai:
Here I plotted for each drug the date it entered jicai and dates of all supplemental filings.
* 45.7% of jicai drugs changed suppliers post-approval
* 16.4% changed production processes
* 15.3% changed manufacturing sites
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
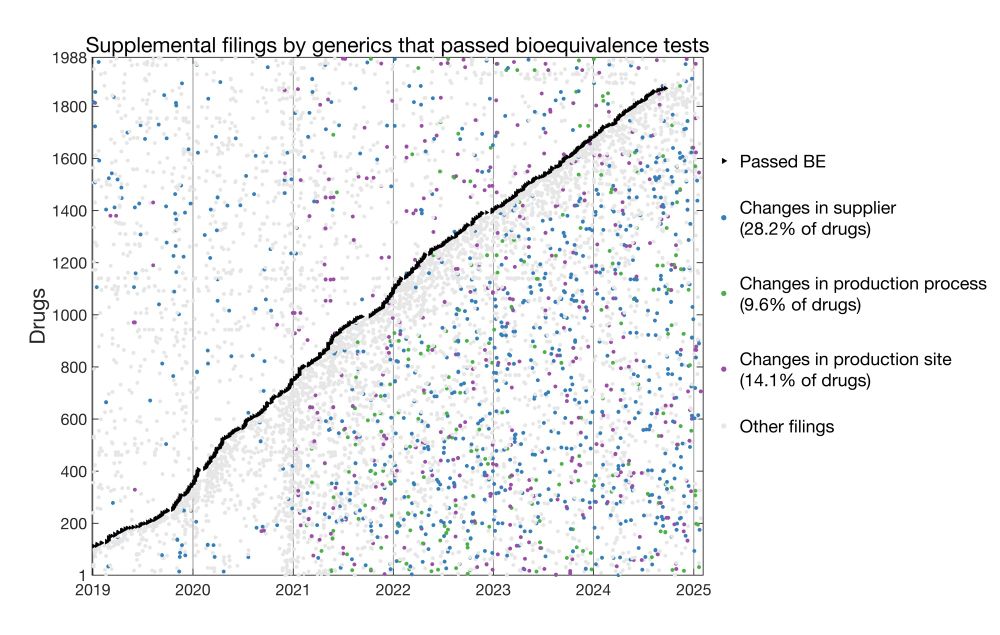
Generics that passed BE:
Here I plotted for each drug the date it passed BE and dates of all supplemental filings.
* 28.2% of generics changed suppliers post-approval
* 9.6% changed production processes
* 14.1% changed manufacturing sites
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
I focused on 3 types of changes: supplier, production process, and manufacturing site. These are more likely to impact drug efficacy than other changes.
Using permit no (国药准字), drug name and manufacturer name, I matched filings to approved generics and jicai drugs.
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
Data source--
Supplemental filings:
www.nmpa.gov.cn/datasearch/s...
Generics that passed BE:
www.cde.org.cn
Jicai drugs: Shanghai division of NHSA
www.smpaa.cn
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
I analyzed supplemental filings disclosed by NMPA during 2019/1/1-2025/2/5 and found a total of >160k filings.
I cross referenced these with generics that passed BE disclosed by NMPA (n = 1,988) and drugs that entered centralized procurement jicai disclosed by NHSA (n = 1,933).
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
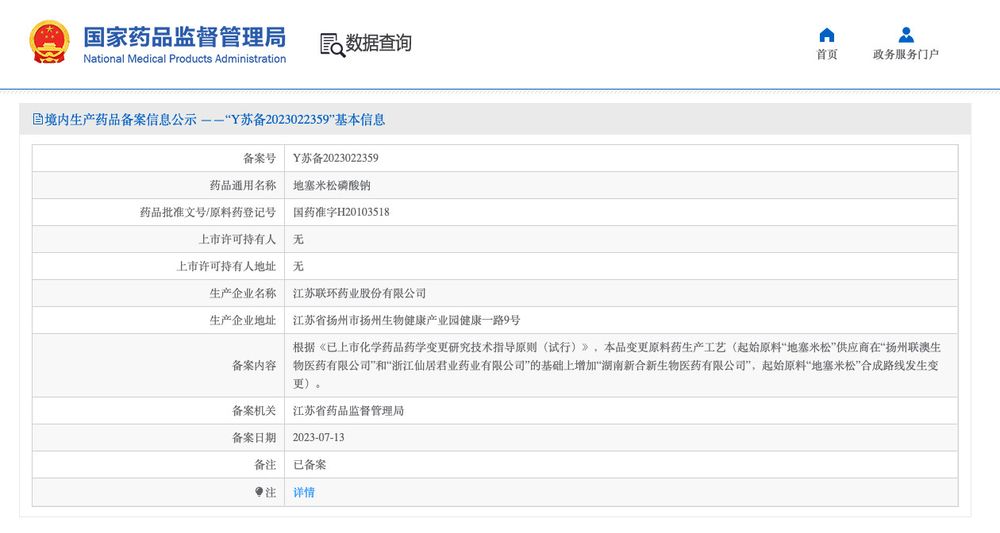

After passing bioequivalence tests, generics can undergo changes in supplier, manufacturing process or site. These are often submitted as supplemental filings to province-level drug admins, and no further BE tests are required.
Here I quantified how prevalent these changes are.
18.02.2025 18:40 — 👍 0 🔁 0 💬 1 📌 0
Thread: prevalence of post-approval changes in generic drugs and jicai (集采) drugs.
I analyzed >160k supplemental filings and found widespread post-approval changes in generics and jicai drugs. Importantly, jicai drugs underwent more changes than non-jicai counterparts.
bsky.app/profile/airm...
18.02.2025 18:40 — 👍 3 🔁 1 💬 1 📌 0
比较干净的药品销量数据可能得靠一些商业数据库了,好像没有什么很好的公开数据
16.02.2025 01:59 — 👍 2 🔁 0 💬 0 📌 0
推广仿制药、集采制度是解决医保支付问题、为广大患者提供低价药品的必要的、有效的方法。
为应对、缓解患者和广大民众对仿制药和集采制度的质疑,药厂、药监部门、医保部门应扩大信息公开的力度并确保公开的信息及时准确。药监部门也应对于可能影响药效、安全性的过评后变更进行更加有效的审核与监管。
16.02.2025 00:53 — 👍 3 🔁 0 💬 0 📌 0
Sr. Engineer for Data Journalism & Investigations @bloomberg.com. Prev. @propublica.org.
Research Scholar at Yale Law School studying the history of science in China and US-China relations. Particle physicist by training. Writer at various places. Editor at Made in China Journal. Co-host of Dissident at the Doorstep.
Senior China reporter, CNN
Tech features and investigations at @technologyreview.com | proud Los Angeles resident | send me tips (not PR pitches) on Signal: eileenguo.15 | português-español-中文 https://www.technologyreview.com/author/eileen-guo/
Data & 📝 @financialtimes.com 📍NYC
Previously @wsj.com, @afpfr.bsky.social covering China
✉️ eva.xiao@ft.com
📱 Signal: @evax.17
Professor of Political Science at Stanford | Exploring money in politics, campaigns and elections, ideology, the courts, and inequality | Author of The Judicial Tug of War cup.org/2LEoMrs | https://data4democracy.substack.com
investigative reporter, professor of data journalism, co-founder, justice media co-lab at boston university.
priors at nytimes, center for public integrity, sd union-trib, inewsource & intercept
we’re all here together, and the weather’s fine ♬ (~);}
Washington Post Investigative Data Reporter
Asia Editor, @NPR.org. Usual caveats.
Author of "Broken Engagement," interviews with top officials on U.S. China policy, and "Superpower Showdown" with
@Lingling_Wei, the definitive account of
Trump's trade war. http://bobdavisreports.com
Assistant Professor at Georgetown • Director of the CFR China Strategy Initiative • Biden NSC China 2021-2024 • Author of The Long Game
New York Times investigative reporter, writing about the world of nonprofits. CNN political analyst. Please send story tips! daf@nytimes.com
Parody. Merch: https://tinyurl.com/msjjmwpt
Geopolitics Editor, author of The Telegram column, The Economist. Previously posted to Beijing, Washington, London, Brussels, Washington, Beijing, Sydney.
Managing Partner, Nüora Global Advisors | Author & Journalist | Vice-chair, NüVoices
https://www.joannachiu.com/
https://nuvoices.com/
Executive Director of the China Media Project, a think tank promoting freedom of expression through research and journalism capacity building.
chinamediaproject.org
https://tianjiancmp.substack.com/
https://linguasinica.substack.com/
Author of Indelible City & The People’s Republic of Amnesia, Host of The Masterclass & Little Red Podcast
senior business & China editor for WIRED. my Signal is louise_matsakis.83. I co-write a newsletter called Made in China: https://www.wired.com/newsletter
Cover tech and geopolitics for the New York Times. Email pmozur at nytimes.com. Check out my past work at https://www.nytimes.com/by/paul-mozur