Death-associated protein kinase 1-dependent SENP1 degradation increases tau #SUMOylation and leads to cognitive dysfunction in a mouse model for #tauopathy
Xindong Shui...Dongmei Chen, Tao Zhang, Tae Ho Lee #AlzheimersDisease
molecularneurodegeneration.biomedcentral.com/articles/10....
21.11.2025 19:30 — 👍 0 🔁 0 💬 0 📌 0

Plasma TDP-43 is a potential biomarker for advanced limbic-predominant age-related TDP-43 encephalopathy neuropathologic change - Molecular Neurodegeneration
#Plasma TDP-43 is a potential #biomarker for advanced limbic-predominant age-related #TDP43 encephalopathy neuropathologic change
Jijing Wang, Julie A. Schneider, David A. Bennett...& Hyun-Sik Yang @harvardmed.bsky.social
molecularneurodegeneration.biomedcentral.com/articles/10....
14.11.2025 18:45 — 👍 0 🔁 0 💬 0 📌 0

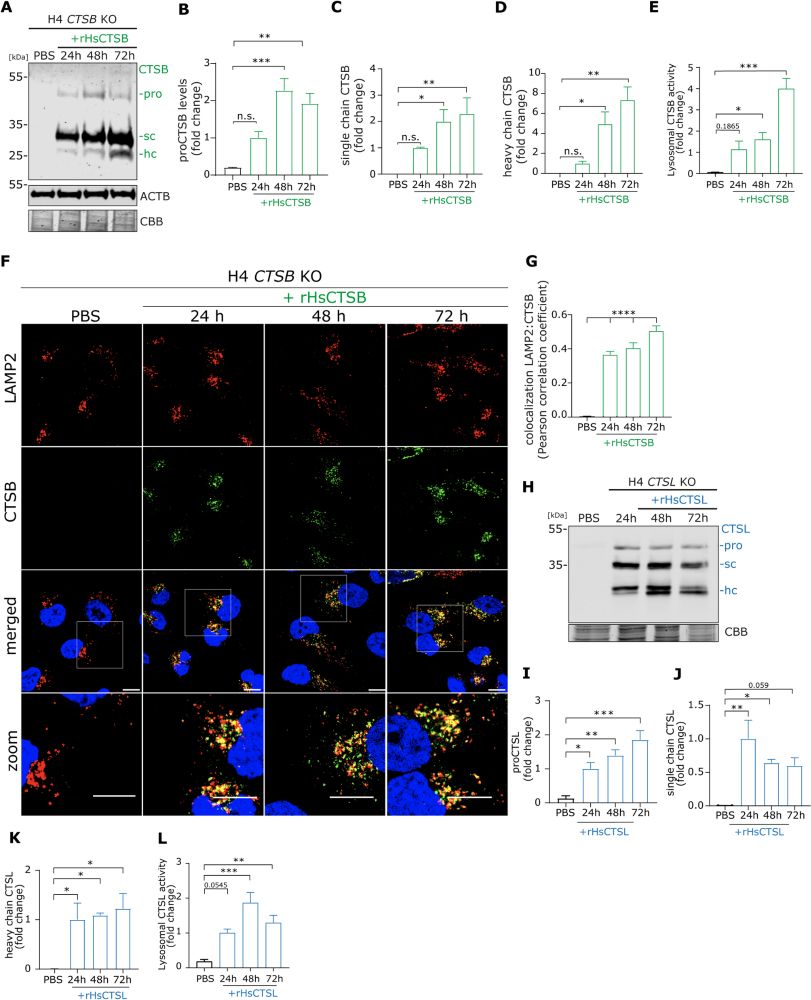
'C-terminus-dependent detection of #lysosomal alpha- #synuclein in nigral #Parkinsons disease human brain neurons'
Martino L. Morella, Bana Al Khayrat, Tim E. Moors...Wilma D. J. van de Berg @amsterdamumc.bsky.social
molecularneurodegeneration.biomedcentral.com/articles/10....
31.10.2025 13:54 — 👍 0 🔁 0 💬 0 📌 0

'Modeling #neurodegeneration in the #retina and strategies for developing pan-neurodegenerative #therapies'
Emily L. Ward...John G. Flanagan @ucberkeleyofficial.bsky.social @harvardmed.bsky.social #Glaucoma #RetinalGanglionCell
molecularneurodegeneration.biomedcentral.com/articles/10....
14.10.2025 14:48 — 👍 0 🔁 0 💬 0 📌 0

'Cerebrospinal fluid markers link to #synapticplasticity responses and #AlzheimersDisease genetic pathways'
Bjørn-Eivind Kirsebom...Kaj Blennow & Tormod Fladby #biomarkers
molecularneurodegeneration.biomedcentral.com/articles/10....
13.10.2025 13:47 — 👍 0 🔁 0 💬 0 📌 0
Client Challenge
'Midbrain degeneration triggers #astrocyte reactivity and tau pathology in experimental #AlzheimersDisease'
Livia La Barbera, Paraskevi Krashia, Gilda Loffredo...Annalisa Nobili & Marcello D’Amelio
molecularneurodegeneration.biomedcentral.com/articles/10....
13.10.2025 13:40 — 👍 0 🔁 0 💬 0 📌 0

Brain somatic mutations in Alzheimer’s disease: linking genetic mosaicism to neurodegeneration - Molecular Neurodegeneration
Somatic mutations are DNA sequence changes that occur in non-reproductive cells during an organism’s life and are not inherited by offspring. Growing evidence implicates somatic mutations in Alzheimer’s disease (AD), linking them to both disease onset and progression. Recent advancements in single-cell sequencing and genome-wide analyses have revealed higher mutation burdens in neurons, particularly in AD-related genes such as Presenilin 1 (PSEN1), Presenilin 2 (PSEN2) and amyloid precursor protein (APP). These mutations, which include single nucleotide variants (SNVs), small insertions and deletions (Indels), structural variations (SVs) and mitochondrial DNA (mtDNA) mutations may disrupt neuronal function and synaptic connectivity. However, some somatic mutations may also serve a neuroprotective role. The underlying mechanisms remain incompletely understood. This review explores the emerging role of somatic mutations in AD, highlighting their links to disease progression. It also underscores the potential for future research to uncover new therapeutic targets by integrating advanced sequencing technologies and gene-editing approaches, which may enable more precise interventions to correct somatic mutations and slow disease progression.
Brain somatic mutations in #AlzheimersDisease: linking #genetic mosaicism to #neurodegeneration
Zuguang Li, Juan Zhang, Zhiqiang Liu...Kai Shu, Ling-Qiang Zhu, Dan Liu
molecularneurodegeneration.biomedcentral.com/articles/10....
09.10.2025 14:25 — 👍 0 🔁 0 💬 0 📌 0

@molneurodegen.bsky.social shorturl.at/B5qbB
Out now.
03.10.2025 13:53 — 👍 0 🔁 1 💬 0 📌 0

Molecular hallmarks of excitatory and inhibitory neuronal resilience to Alzheimer’s disease - Molecular Neurodegeneration
Background A significant proportion of individuals maintain cognition despite extensive Alzheimer’s disease (AD) pathology, known as cognitive resilience. Understanding the molecular mechanisms that protect these individuals could reveal therapeutic targets for AD. Methods This study defines molecular and cellular signatures of cognitive resilience by integrating bulk RNA and single-cell transcriptomic data with genetics across multiple brain regions. We analyzed data from the Religious Order Study and the Rush Memory and Aging Project (ROSMAP), including bulk RNA sequencing (n = 631 individuals) and multiregional single-nucleus RNA sequencing (n = 48 individuals). Subjects were categorized into AD, resilient, and control based on β-amyloid and tau pathology, and cognitive status. We identified and prioritized protected cell populations using whole-genome sequencing-derived genetic variants, transcriptomic profiling, and cellular composition. Results Transcriptomics and polygenic risk analysis position resilience as an intermediate AD state. Only GFAP and KLF4 expression distinguished resilience from controls at tissue level, whereas differential expression of genes involved in nucleic acid metabolism and signaling differentiated AD and resilient brains. At the cellular level, resilience was characterized by broad downregulation of LINGO1 expression and reorganization of chaperone pathways, specifically downregulation of Hsp90 and upregulation of Hsp40, Hsp70, and Hsp110 families in excitatory neurons. MEF2C, ATP8B1, and RELN emerged as key markers of resilient neurons. Excitatory neuronal subtypes in the entorhinal cortex (ATP8B+ and MEF2Chigh) exhibited unique resilience signaling through activation of neurotrophin (BDNF-NTRK2, modulated by LINGO1) and angiopoietin (ANGPT2-TEK) pathways. MEF2C+ inhibitory neurons were over-represented in resilient brains, and the expression of genes associated with rare genetic variants revealed vulnerable somatostatin (SST) cortical interneurons that survive in AD resilience. The maintenance of excitatory-inhibitory balance emerges as a key characteristic of resilience. Conclusions We have defined molecular and cellular hallmarks of cognitive resilience, an intermediate state in the AD continuum. Resilience mechanisms include preserved neuronal function, balanced network activity, and activation of neurotrophic survival signaling. Specific excitatory neuronal populations appear to play a central role in mediating cognitive resilience, while a subset of vulnerable interneurons likely provides compensation against AD-associated hyperexcitability. This study offers a framework to leverage natural protective mechanisms to mitigate neurodegeneration and preserve cognition in AD.
Thrilled to share that our paper “Molecular hallmarks of excitatory and inhibitory neuronal resilience to Alzheimer’s disease” is now out in Molecular Neurodegeneration!
👉 link.springer.com/article/10.1...
#Alzheimer #Resilience #Neuroscience
01.10.2025 19:45 — 👍 16 🔁 6 💬 1 📌 1

Molecular hallmarks of excitatory and inhibitory neuronal resilience to Alzheimer’s disease - Molecular Neurodegeneration
Background A significant proportion of individuals maintain cognition despite extensive Alzheimer’s disease (AD) pathology, known as cognitive resilience. Understanding the molecular mechanisms that protect these individuals could reveal therapeutic targets for AD. Methods This study defines molecular and cellular signatures of cognitive resilience by integrating bulk RNA and single-cell transcriptomic data with genetics across multiple brain regions. We analyzed data from the Religious Order Study and the Rush Memory and Aging Project (ROSMAP), including bulk RNA sequencing (n = 631 individuals) and multiregional single-nucleus RNA sequencing (n = 48 individuals). Subjects were categorized into AD, resilient, and control based on β-amyloid and tau pathology, and cognitive status. We identified and prioritized protected cell populations using whole-genome sequencing-derived genetic variants, transcriptomic profiling, and cellular composition. Results Transcriptomics and polygenic risk analysis position resilience as an intermediate AD state. Only GFAP and KLF4 expression distinguished resilience from controls at tissue level, whereas differential expression of genes involved in nucleic acid metabolism and signaling differentiated AD and resilient brains. At the cellular level, resilience was characterized by broad downregulation of LINGO1 expression and reorganization of chaperone pathways, specifically downregulation of Hsp90 and upregulation of Hsp40, Hsp70, and Hsp110 families in excitatory neurons. MEF2C, ATP8B1, and RELN emerged as key markers of resilient neurons. Excitatory neuronal subtypes in the entorhinal cortex (ATP8B+ and MEF2Chigh) exhibited unique resilience signaling through activation of neurotrophin (BDNF-NTRK2, modulated by LINGO1) and angiopoietin (ANGPT2-TEK) pathways. MEF2C+ inhibitory neurons were over-represented in resilient brains, and the expression of genes associated with rare genetic variants revealed vulnerable somatostatin (SST) cortical interneurons that survive in AD resilience. The maintenance of excitatory-inhibitory balance emerges as a key characteristic of resilience. Conclusions We have defined molecular and cellular hallmarks of cognitive resilience, an intermediate state in the AD continuum. Resilience mechanisms include preserved neuronal function, balanced network activity, and activation of neurotrophic survival signaling. Specific excitatory neuronal populations appear to play a central role in mediating cognitive resilience, while a subset of vulnerable interneurons likely provides compensation against AD-associated hyperexcitability. This study offers a framework to leverage natural protective mechanisms to mitigate neurodegeneration and preserve cognition in AD.
'Molecular hallmarks of excitatory and inhibitory neuronal resilience to #AlzheimersDisease'
Isabel Castanho, Pourya Naderi Yeganeh...Rudolph E. Tanzi & Winston Hide @winhide.bsky.social @harvardmed.bsky.social
#CognitiveResilience #transcriptomics #genetics
bit.ly/3VK7k5b
01.10.2025 14:29 — 👍 4 🔁 1 💬 0 📌 0

✅ Our "Emerging Insights into #LewyBodyDisease" review series is now complete!
Dive into the full collection covering diagnosis, management & mechanisms of LBD
Explore all articles here ➡️ biomedcentral.com/collections/...
#synucleinopathies #Parkinsons #microbiome #neuroimaging #sleep
30.09.2025 15:03 — 👍 2 🔁 1 💬 0 📌 0

#Microglia networks within the tapestry of #AlzheimersDisease through spatial #transcriptomics
Yi Zhou & Christopher K. Glass @ucsandiego.bsky.social
molecularneurodegeneration.biomedcentral.com/articles/10....
29.09.2025 19:23 — 👍 1 🔁 2 💬 0 📌 0

Making tracks: microglia and the extracellular matrix - Molecular Neurodegeneration
Microglia are resident immune cells of the central nervous system (CNS) and critical regulators of neural homeostasis, mediating immune surveillance, synaptic remodeling, debris clearance, and inflammatory signaling. Emerging evidence highlights the extracellular matrix (ECM) as important to microglial behavior in both physiological and pathological contexts. The CNS ECM is a dynamic and bioactive scaffold composed of three primary compartments: interstitial matrix, basement membranes at neurovascular and neuroepithelial interfaces, and perineuronal nets (PNNs). Each compartment exhibits distinct molecular architectures, ranging from fibrillar collagens and glycoproteins in basement membranes to chondroitin sulfate proteoglycans and hyaluronan-rich structures in PNNs. In this review we examine how microglia engage with and reshape the ECM to dynamically respond to disruptions in homeostasis with aging and disease. We discuss the concept of the microglial–ECM “interactome”, which may represent a molecular interface through which microglia sense, modify, and respond to their extracellular environment. This interactome enables microglia to enact fine-scale ECM remodeling during routine surveillance, as well as large-scale alterations under pathological conditions to help preserve function and motility. In aging and disease, dysregulation of the microglial-ECM interactome is characterized by aberrant mechanotransduction, elevated proteinase activity, remodeling of the ECM, and sustained pro-inflammatory cytokine release. These pathological changes compromise ECM integrity, challenge microglial activity, and contribute to progressive neurovascular and synaptic dysfunction. Deciphering the molecular mechanisms underpinning microglial–ECM interactions is essential for understanding region-specific vulnerability in neurodegeneration and may reveal new therapeutic targets for preserving ECM structure and countering CNS disorders.
'Making tracks: #microglia and the #ExtracellularMatrix'
Lauren K. Wareham & David J. Calkins #neurodegeneration
molecularneurodegeneration.biomedcentral.com/articles/10....
29.09.2025 15:59 — 👍 1 🔁 0 💬 0 📌 0

The Hippo signaling pathway as a therapeutic target in Alzheimer’s disease - Molecular Neurodegeneration
The Hippo signaling pathway is well-known for its regulation of organ size, cell proliferation, apoptosis, and cell migration and differentiation. Recent studies have demonstrated that Hippo signaling also plays important roles in the nervous system, being involved in neuroinflammation, neuronal differentiation, and neuronal death and degeneration. As such, dysregulation of Hippo signaling, particularly of its core kinases MST1/2 and LATS1/2, has begun to attract attention in the Alzheimer’s disease (AD) field. Here, we discuss the therapeutic potential of targeting the Hippo pathway in AD by providing an overview of Hippo signaling with regards to its function in the nervous system, evidence for its dysregulation in AD patients and models, and recent studies involving genetic or pharmacological modulation of this pathway in AD.
The #HippoSignaling pathway as a #therapeutic target in #AlzheimersDisease
Doris Chen, Stella Wigglesworth-Littlewood, Frank J. Gunn-Moore
molecularneurodegeneration.biomedcentral.com/articles/10....
28.09.2025 16:51 — 👍 0 🔁 0 💬 0 📌 0

Autophagic impairment in sleep–wake circuitry is linked to sleep loss at the early stages of Alzheimer’s disease - Molecular Neurodegeneration
Background Proteostasis, in particular the impairment of autophagic activity, is linked to sleep dysregulation and is an early sign of dementias including Alzheimer’s disease (AD). This coupling of events may be a critical alteration driving proteinopathy and AD progression. In the present study, we investigated sleep–wake and memory regulating neurons for vulnerability to autophagic impediment, and related these findings to progression of the sleep and cognitive phenotype. Methods Using the double knock-in AD mouse model, AppNL−G−FxMAPT, we examined phenotypic and pathological alterations at several timepoints and compared to age-matched single knock-in MAPT mice. Spatial learning, memory and executive Function were investigated in the Barnes maze. Sleep was investigated by 24-h locomotor activity and EEG. Immunostaining for autophagic, neuronal and pathological markers was conducted in brain regions related to memory (hippocampus, prefrontal cortex, entorhinal cortex) and the sleep–wake cycle (hypothalamus, locus coeruleus). Hippocampal electrophysiological recordings were conducted to probe neuronal Function during object investigation. A 3-day sleep disruption was conducted in MAPT mice to investigate autophagic changes following sleep loss. Autophagy was activated in MAPT mice with trehalose to probe effects on sleep recovery. Results We identified that disrupted sleep occurred from early-stages in AppNL−G−FxMAPT mice, that sleep declined over age, and sleep deficits preceded cognitive impairments in late-stages. Cytoplasmic autophagic impediment in hypothalamic and locus coeruleus sleep–wake neurons occurred in early-stage AppNL−G−FxMAPT mice, prior to significant β-amyloid deposition in these regions, with a failure of lysosomal flux over disease progression. Autophagic changes in the hippocampus and cortex at early-stage were predominantly in processes and less frequently associated with the lysosome. Plaque-associated autophagic and lysosomal accumulations were frequent from the early-stage. Sex differences in the AD phenotype were prominent, including greater cognitive decline in males than females, linked to increased proteostasis burden in EC layer II neurons and hippocampal tau in the late-stage. Conversely, sleep impairments were more rapid in females including less REM sleep recovery than males, along with greater autophagic burden in hippocampal processes of female AppNL−G−FxMAPT mice. We probed the sleep-cognition linkage demonstrating hippocampal electrophysiological slowing during cognitive processing in mid-stage AppNL−G−FxMAPT mice, prior to cognitive decline. We provide evidence for a positive feedback loop in the autophagic-sleep relationship by demonstrating that disrupted sleep in MAPT mice led to arrhythmic sleep patterns and accumulations of autophagic aggregates in the hippocampus and hypothalamus, similar to as was seen in the early Alzheimer’s phenotype. We further probed the autophagy-sleep linkage by treating MAPT mice with trehalose to activate autophagy and demonstrate an improvement in sleep recovery following a sleep disruption. Conclusions These findings demonstrate the vulnerability of sleep-regulating neurons to proteostatic dysfunction and the sleep-autophagy linkage as an early, and treatable, Alzheimer’s disease mechanism. Graphical Abstract Morrone et al provide evidence for the linkage between sleep and autophagic disruptions in Alzheimer’s disease (AD) progression. At early AD stages, sleep-wake regulating neurons in the hypothalamus and locus coeruleus exhibit increased cytoplasmic inclusions concomitant with the onset of sleep disturbances. Early-stage autophagic aggregates in the hippocampus appear more prominently in neuronal processes and in the cortex linked to plaques. This pathology worsens over AD progression, including advanced sleep and cognitive deficits, autophagic aggregates in entorhinal cortex-hippocampus projecting neurons. Disrupting sleep in control mice mimics the hippocampal, hypothalamic and sleep patterns impairments observed in early-stage AD, and therapeutic activation of autophagy improves sleep recovery. See also Table 1 for a summary of changes along with sex differences in autophagy and behavioral readouts.
#Autophagic impairment in #sleep–wake circuitry is linked to sleep loss at the early stages of #AlzheimersDisease
Christopher Daniel Morrone, Arielle A. Tsang, W. Haung Yu
molecularneurodegeneration.biomedcentral.com/articles/10....
28.09.2025 16:50 — 👍 0 🔁 0 💬 0 📌 0

A novel alpha-synuclein G14R missense variant is associated with atypical neuropathological features - Molecular Neurodegeneration
Background Parkinson’s disease (PD) affects millions of people worldwide, but only 5–10% of patients suffer from a monogenic forms of the disease with Mendelian inheritance. SNCA, the gene encoding for the protein alpha-synuclein (aSyn), was the first to be associated with familial forms of PD and, since then, several missense variants and multiplications of the gene have been established as rare causes of autosomal dominant forms of PD. In this study, we report the identification of a novel SNCA mutation in a patient that presented with a complex neurogenerative disorder, and unconventional neuropathological findings. We also performed in depth molecular studies of the effects of the novel aSyn mutation. Methods A patient carrying the novel aSyn missense mutation and the family members were studied. We present the clinical features, genetic testing—whole exome sequencing (WES), and neuropathological findings. The functional consequences of this aSyn variant were extensively investigated using biochemical, biophysical, and cellular assays. Results The patient exhibited a complex neurodegenerative disease that included generalized myocloni, bradykinesia, dystonia of the left arm and apraxia. WES identified a novel heterozygous SNCA variant (cDNA 40G > A; protein G14R). Neuropathological examination showed extensive atypical aSyn pathology with frontotemporal lobar degeneration (FTLD)-type distribution and nigral degeneration pattern with abundant ring-like neuronal inclusions, and few oligodendroglial inclusions. Sanger sequencing confirmed the SNCA variant in one healthy, 86-year-old parent of the patient suggesting incomplete penetrance. NMR studies suggest that the G14R mutation induces a local structural alteration in aSyn, and lower thioflavin T binding in in vitro fibrillization assays. Interestingly, the G14R aSyn fibers display different fibrillar morphologies than Lewy bodies as revealed by cryo-electron microscopy. Cellular studies of the G14R variant revealed increased inclusion formation, enhanced membrane association, and impaired dynamic reversibility of serine‐129 phosphorylation. Conclusions The atypical neuropathological features observed, which are reminiscent of those observed for the G51D aSyn variant, suggest a causal role of the SNCA variant with a distinct clinical and pathological phenotype, which is further supported by the properties of the mutant aSyn.
'A novel alpha- #synuclein G14R missense variant is associated with atypical #neuropathological features'
Christof Brücke, Mohammed Al-Azzani...Tiago Fleming Outeiro
molecularneurodegeneration.biomedcentral.com/articles/10....
28.09.2025 16:49 — 👍 0 🔁 0 💬 0 📌 0
A community of more than 250 MIT researchers dedicated to understanding the fundamental mechanisms of how the brain works and applying that insight to disease.
Neuroscientist passionate about astrocytes and the brain vasculature. Group leader at @UKDRI - @EdinburghUni. (she/her)
https://www.diaz-castrolab.com
She/her
Postdoc-Temple Lab (@neuralstemcells.bsky.social)
Interested in immune-vascular interactions in AD. Passionate about mentoring and glia.
💜
IPSCs/🧠/🧶/🎂/🐈
Joint Head of Structural Studies at @mrclmb.bsky.social. Develops & uses #cryoEM to study amyloids in neurodegeneration. #tau, #alphasynuclein, #opensoftware, #RELION. All opinions my own.
Led by Beth Mormino @ Stanford. We investigate aging and neurodegenerative disease in preclinical populations utilizing biofluid and imaging biomarkers, fMRI and MRI. 🧠
https://med.stanford.edu/mormino-lab.html
PI & Associate Professor @Karolinska Institute | Research Affiliate @Mayo Clinic | Director of Center for Alzheimer Research
Incoming Assistant Professor @RutgersU Cell Bio & Neuro 👩🏻🔬
Neurodegeneration, tau, and things in between 🧠
Postdoc @bcmhouston / PhD @MayoClinic 🥼
**views my own
🔗 https://dcchunglab.com
An aging neuroscientist (in every sense of that phrase). Respects science and logic. Does not suffer fools gladly. Progressive in outlook. Member Scotch Malt Whiskey Society. You should taste my smoked brisket.
Interdisciplinary virtual network of ~850 neuroscientists at the University of Cambridge and affiliated Institutes.
www.neuroscience.cam.ac.uk
A grassroots movement turned non-profit organization with a mission to diversify the neurosciences by building a community that celebrates and empowers Black scholars and professionals in neuroscience-related fields.
The Parkinson's Foundation knows that a research breakthrough can happen at any time & believes in educating our PD community with the latest research updates.
https://www.parkinson.org/advancing-research
AWI at Boston University School of Public Health mission is to transform lives, families, communities and populations by converting research and science to real world solutions.
We are shaping the future of genomic research, medicine, and education in the largest, most diverse healthcare system in New York City. 🧬
https://www.flowcode.com/page/sinaigenetics
Department of Neurological Surgery @ucsanfrancisco.bsky.social. We focus on providing the best possible care for our patients, alongside research efforts to develop better treatments.
neurosurgery.ucsf.edu
Cell aims to publish the most exciting and provocative research in biology. Posts by Scientific Editors on the Cell editorial team. See the latest papers at https://www.cell.com/cell/
News, commentary and research coverage from the international monthly journal publishing the highest quality of work in all areas of neuroscience.
https://www.nature.com/neuro/
Research, news, and commentary from Nature, the international science journal. For daily science news, get Nature Briefing: https://go.nature.com/get-Nature-Briefing
Neuron publishes ground-breaking research papers, reviews & commentary across neuroscience and is a premier intellectual forum for the neuroscience community.
https://www.cell.com/neuron/home
We're the Crick, a biomedical research lab in London working to figure out how life works.
Home to more than 2,000 scientists and a free public exhibition space.
https://www.crick.ac.uk/
Neuroscientist 🧠👩🏼🔬, UCLA, studying the brain circuits of learning, decision making, and habits.