More on Elysis and their cleaner Alum. smelting process. Interesting that it increases energy used, because you aren't getting the chemical energy from the carbon. Thus, it is more more vital to use clean power.
www.canarymedia.com/articles/cle...
25.11.2025 15:28 — 👍 15 🔁 4 💬 1 📌 1

An electrifying new ironmaking method could slash carbon emissions
By extracting metallic iron without producing carbon dioxide, the new process could even be carbon negative, at least for part of the world’s iron production
It is possible to make steel without any combustion of fossil fuels or process emissions. The magic? Electrochemistry!
It will take a while to bring down costs, but this is inevitable. It makes too much sense.
30.10.2025 17:42 — 👍 160 🔁 43 💬 6 📌 2

Last week we hosted the 5th Annual Conference of the Oregon Center for Electrochemistry in conjunction with the Electrochemical Society Pacific Northwest Section Fall Meeting!
Great way to kick off the fall quarter with a new cohort of electrochemistry students here at Oregon.
30.09.2025 21:27 — 👍 1 🔁 0 💬 0 📌 0
Is this a known hallucination? I assumed you pulled this out of a hat and it's kind of inexplicable but I guess that's the whole point.
08.08.2025 18:14 — 👍 2 🔁 0 💬 1 📌 0

Lol
08.08.2025 18:13 — 👍 6 🔁 1 💬 1 📌 0
A joint student with @boettcherlab.bsky.social she also kept the lab safe and mentored our first two electrochemistry 4+1 masters students Caitlyn and Nadia in their freshman research project (shown above as proud mentees).
09.05.2025 19:17 — 👍 2 🔁 0 💬 0 📌 0

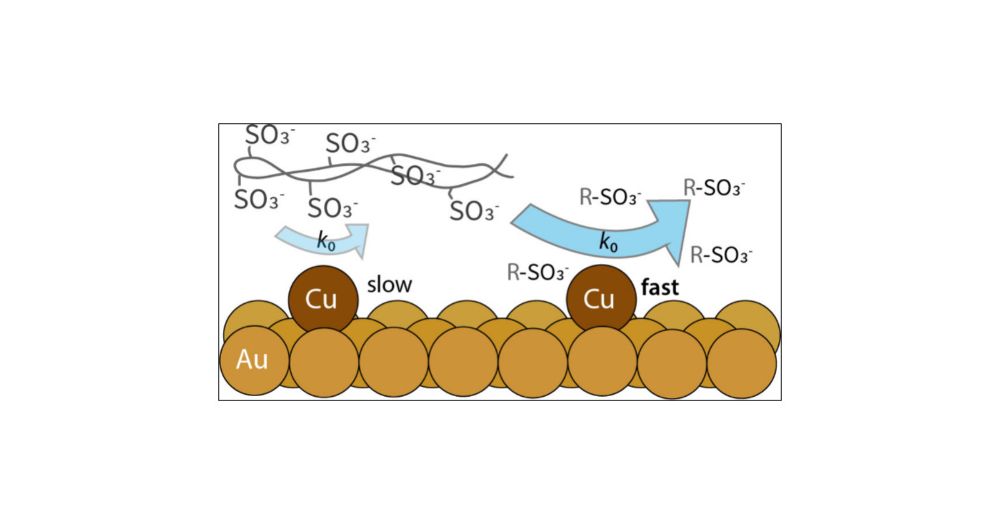
Congratulations to the newly minted Dr. Thurman and the first Ph.D. from the Kempler group! Kira taught us about the influence of solvent and electrolyte on copper corrosion and deposition kinetics.
09.05.2025 19:17 — 👍 4 🔁 0 💬 2 📌 0
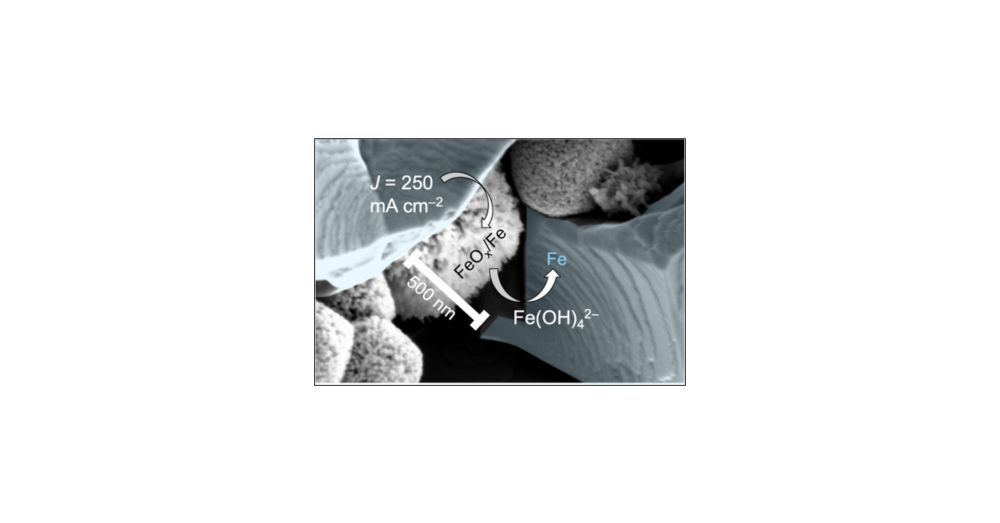
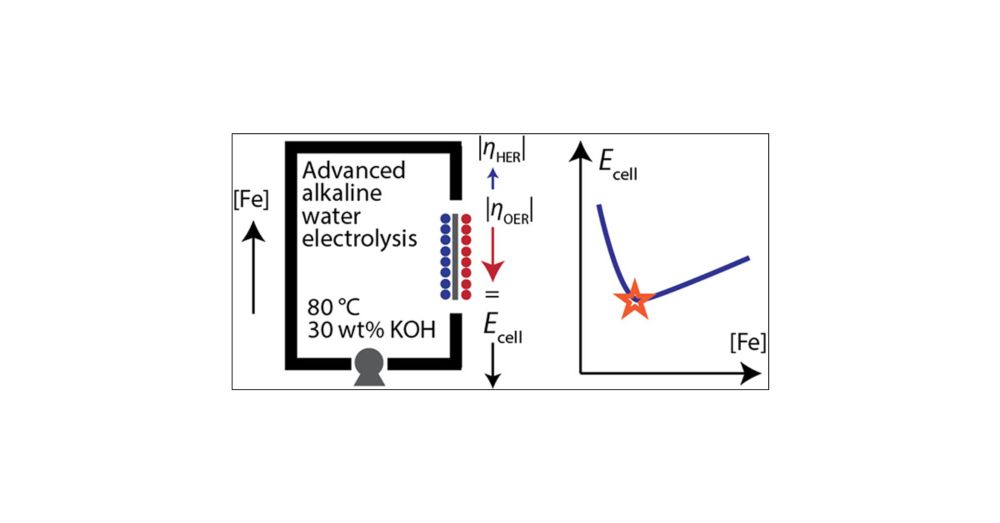
Read about our latest efforts to understand what controls the maximum rate of iron metal production from oxides... and what it means for the path to affordable green steel: pubs.acs.org/doi/full/10.... congrats to Ana, Andrew and the rest of the team.
09.04.2025 17:08 — 👍 3 🔁 0 💬 0 📌 0
As well as whether or not any of the vanillin comes from vanilla beans (mostly not?)
03.04.2025 01:51 — 👍 0 🔁 0 💬 1 📌 0

SMASH Engineering REU
Notre Dame Materials Science and Engineering aims to promote the interdisciplinary understanding of materials through collaborative research to advance knowledge and promote the greater good.
SMASH Engineering REU (Soft Materials for Applications in Sustainability and Healthcare Engineering Research Experience for Undergraduates) site program is accepting applications for summer 2025! Please share this opportunity!!
mse.nd.edu/research/sma...
18.03.2025 11:41 — 👍 4 🔁 3 💬 0 📌 0

Are you a chemist currently working in industry? We are interested in hearing about your experience. Please see the flyer for more information, we would love to talk with you!
05.02.2025 21:13 — 👍 6 🔁 4 💬 1 📌 2
Fe effects remain important at high temperature and pH, and while Fe improves the intrinsic activity of metal (oxy)hydroxide catalyst films it can limit the active catalyst sites. Optimum concentrations are within a range that could be controlled by the BOP.
03.02.2025 21:20 — 👍 1 🔁 0 💬 0 📌 0
Class of 2025 – Oregon Center for Electrochemistry
(Electro)chemsky! These students are available for hire beginning April or June 2025 and do not have to return to Eugene after completing their internship (electrochemistry.uoregon.edu/masters-inte...)
Let us know if you'd like to see some resumes and please share for visibility 🧪⚡
14.01.2025 19:36 — 👍 11 🔁 5 💬 0 📌 0
M.S. Internship Program Flyer – Oregon Center for Electrochemistry
Applications are open for the 6th cohort of the Electrochemistry Masters Internship Program! If you (or any undergraduates you know) are interested in a cleantech career related to electrochemistry this is a unique opportunity: electrochemistry.uoregon.edu/masters-inte... ⚡chemsky
27.11.2024 00:44 — 👍 23 🔁 9 💬 0 📌 0
Here is a new starter pack to follow some electrochemists (More to come)
go.bsky.app/UzqjkNk
25.11.2024 03:53 — 👍 32 🔁 12 💬 6 📌 0
Starter pack for researchers at the University of Oregon. I was expecting a bunch more, to be frank. People might not be listing their affiliations, so please do let me know if you would like to be added. go.bsky.app/HNkp5Uq
25.11.2024 02:36 — 👍 15 🔁 4 💬 1 📌 0

Stern (no relation to the Stern layer) presenting on their fundamental studies on interfacial ion transfer
23.11.2024 00:41 — 👍 1 🔁 0 💬 0 📌 0

Doran Pennington talking about @chhendon.bsky.social lab research on the electrochemistry of coffee
23.11.2024 00:22 — 👍 3 🔁 0 💬 1 📌 0

Andrew Goldman kicking things off by talking about all the things electrochemistry can do (interface with biology, clean water, make metals, make fuels) beyond charging batteries
23.11.2024 00:19 — 👍 0 🔁 0 💬 1 📌 0

Students in a classroom discussing electrochemistry
Packed house for the first ever Electrochemical Society student chapter meeting at the University of Oregon! chemsky 🧪 electrochemsky ⚡
23.11.2024 00:07 — 👍 16 🔁 1 💬 1 📌 0
No 🔋⚡ELECTROCHEMSKY⚡🔋 starter pack? That's fine, we'll make our own.
go.bsky.app/7BokNKP
12.11.2024 15:20 — 👍 52 🔁 21 💬 11 📌 7
Here's a starter pack of engineers (and a few scientists) working in Clean Tech R&D. Let me know if you'd like to be included! #EnergySky #GreenSky #Science 🧪🔌💡
go.bsky.app/2Um4Ubc
18.11.2024 05:28 — 👍 166 🔁 33 💬 17 📌 1

Hi 🦋 chemsky! I'm an assistant prof teaching/researching electrochemistry at the University of Oregon.
Currently curious about conversion reactions between metals/metal oxides... particularly for green steel, aqueous batteries, and electrolyzers powered by intermittent power ⚡
18.11.2024 05:52 — 👍 6 🔁 1 💬 1 📌 0
Delaware MSE faculty leading FINCH Lab growing oxide & chalcogenide films; formerly Auburn & PNNL; Carnegie Mellon & UVA alum/sports fan. Opinions here are mine, not my employer's. There will be a lot of politics. He/him.
http://sites.udel.edu/finch-lab
Science, Chemistry and Gin Tonics, Master of Zombies, Professor of Energy at Newcastle University #RS_ResearchFellow #URF #WomenInSTEM #WomenInChemistry #Berliner #firstgen EDI+ Fellow @BerryCells
optimistic nihilist
www.freitaglab.com
Group leader @ Helmholtz Zentrum Berlin
Website https://www.helmholtz-berlin.de/electroconversion
electrocatalysis, CO2, CCU, photoelectrochemistry
Gscholar https://scholar.google.com/citations?hl=en&user=Y5CgyWcAAAAJ
No longer in good standing with the New York State bar
My newsletter: StringinaMaze.net
Freelance science and technology journalist. 11+ years experience. Irish/Polish. I write for the BBC, Wired, the BMJ, The Guardian, and others!
Newsletter: thereengineer.pro
Portfolio: chrisbaraniuk.com
HQ: Belfast, Northern Ireland
Assistant Professor University of Oregon Sociology | Former Postdoc NYU CSMaP | Ph.D. Princeton Sociology | Research on media, information, politics, China, computational social science | Opinions are my own | https://hwaight.github.io/
Menswear writer. Editor at Put This On. Words at The New York Times, The Washington Post, The Financial Times, Esquire, and Mr. Porter.
If you have a style question, search:
https://dieworkwear.com/ | https://putthison.com/start-here/
Mountains, paddleboards & bikes. Tech & policy towards a net-zero GHG economy, focus on industry. Happy globalist. @ColumbiaUEnergy @bataille_chris
http://IDDRI.org, http://netzerosteel.org http://netzeroindustry.org IPCC AR7 CLA WGIII
Ex NY Times, now author of Substack Paul Krugman. Nobel laureate and, according to Donald Trump, "Deranged BUM". Also Research Professor at the City University of New York Graduate Center and senior scholar at the GC-CUNY Stone Center.
chemist, materials scientist
Improving all lives through the transforming power of chemistry
ACS Publications is proud to publish the most trusted, most cited, and most read collection of journals in the chemical and related sciences.
Explore ACS Publications, a division of @acs.org: pubs.acs.org
Now: decarbonizing the electricity sector with
@rockymtninst.bsky.social | Then: @INDems @HawaiiSenate | Always: @UCBerkeley | all views are my future dog’s
JFD (he/him) & research | analytical & materials chemistry | creighton university www.destinolab.org
A unique forum for the publication of innovative, cutting-edge research on the development of alternative green and sustainable chemistry systems, methods, and technologies.
Published by @rsc.org 🌐 Website: rsc.li/greenchemistry
Urgent short communications of outstanding significance from across the chemical sciences. Email: chemcomm-rsc@rsc.org
Published by @rsc.org 🌐 Website: rsc.li/chemcomm-journal
Flagship journal for @rsc.org, open access with no publication fee, all topics in chemistry. chemicalscience-rsc@rsc.org 🌐 Website: rsc.li/chemscience
U.S. Senator for Oregon. Rotisserie chicken enthusiast. Senate’s resident privacy hawk.
senatefinance.bsky.social
wydenpress.bsky.social
Astronomer here! I’m a Hungarian-American radio astronomer, alias /r/Andromeda321 on Reddit. Assistant professor at the University of Oregon