
Interested in the biophysics of organoids? We just published a review in Dev Cell—take a look! dlvr.it/TPyTb8 #Organoids #Biophysics
06.01.2026 10:27 — 👍 81 🔁 26 💬 0 📌 2@pflenne.bsky.social
Biophysicist interested in cell dynamics and tissue morphogenesis at IBDM and Turing Center for Living Systems Group: https://www.morphotiss.org/ https://www.ibdm.univ-mrs.fr/physical-approaches-to-cell-dynamics/ https://centuri-livingsystems.org

Interested in the biophysics of organoids? We just published a review in Dev Cell—take a look! dlvr.it/TPyTb8 #Organoids #Biophysics
06.01.2026 10:27 — 👍 81 🔁 26 💬 0 📌 2Appreciate Quanta for shining a light on our joint work with Simon Gsell, Sham Tlili (@shamtlili.bsky.social), and Matthias Merkel (@merkellab.bsky.social).
11.10.2025 07:04 — 👍 36 🔁 14 💬 2 📌 1Mechanosensation and fast reorientation of ciliary structures: Marvin's work on the surprising locomotion capabilities of Trichoplax, an animal without neurons!
www.biorxiv.org/content/10.1...
Katia took the initiative to start a Xenopus project in the lab a few years ago —and now her work is highlighted by Development! Huge congrats, Katia!
31.07.2025 10:39 — 👍 32 🔁 7 💬 0 📌 0Feat. @guignardlab.bsky.social @pflenne.bsky.social @shamtlili.bsky.social @philipperoudot.bsky.social
The Tapenade pipeline is fully open-source with user-friendly notebooks and Napari plugins: github.com/GuignardLab/... 🫒

🥇Sham Tlili has been awarded the CNRS 2025 Bronze Medal! She studies how physical forces shape living tissues by using mouse stem cell models known as gastruloids. A unique blend of physics & biology.
🔗 Read more: www.ibdm.univ-amu.fr/sham-tlili-w...
Thank you so much for sharing your thoughts on our article!
10.04.2025 18:33 — 👍 11 🔁 0 💬 1 📌 0

Excited to share that IBDM (Institut de Biologie du Développement de Marseille) is expanding its research groups this year! A great place for developmental biology and interdisciplinary research located on the beautiful Marseille-Luminy campus!
Deadline for applications: March 30th, 2025
Hi Romain, Thanks for noticing it. I transferred some old Twitter posts to BlueSky, but it didn't work that well.
Code: data.mendeley.com/datasets/78n...
Ref: www-nature-com.insb.bib.cnrs.fr/articles/s41...
Congrats on this beautiful work!
15.12.2024 14:21 — 👍 1 🔁 0 💬 1 📌 0Thrilled that our team clinched both 1st and 2nd place in the image contest! 🥇🥈 Huge applause for everyone’s creativity and excitement in front of the microscope. Let’s keep inspiring each other! 💪✨ #Teamwork #Science
15.12.2024 14:17 — 👍 9 🔁 1 💬 0 📌 0And usually, the sky is blue in Cassis, near Marseille!
19.11.2024 07:35 — 👍 5 🔁 0 💬 0 📌 0
Vikas Trivedi's @LabTrivedi @EMBLBarcelona @the_prbb and my group @Equipe_lenne @IBDMmarseille @centuri_ls are recruiting for a Postdoc project on the emergence of mechano-genetic patterns using embryonic organoids!
Start Date: Feb 2023 or later
Informal inquiries are welcome!
How do cell-cell contacts remodel in vivo? We address this question here:
biorxiv.org/content/10.110…
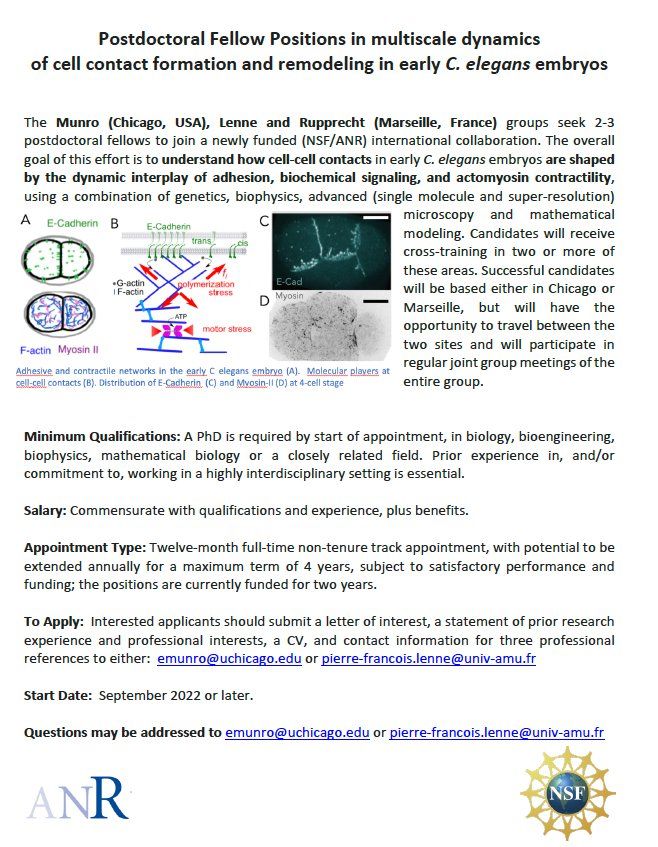
The Munro (Chicago), Lenne and Rupprecht (Marseille) groups seek 2-3 postdoctoral fellows to join a newly funded (NSF/ANR) international collaboration on the multiscale dynamics of cell contact formation and remodeling.
10.05.2022 12:59 — 👍 15 🔁 21 💬 1 📌 0Our work on endoderm formation in gastruloids is out!@eLife
doi.org/10.7554/eLife.…
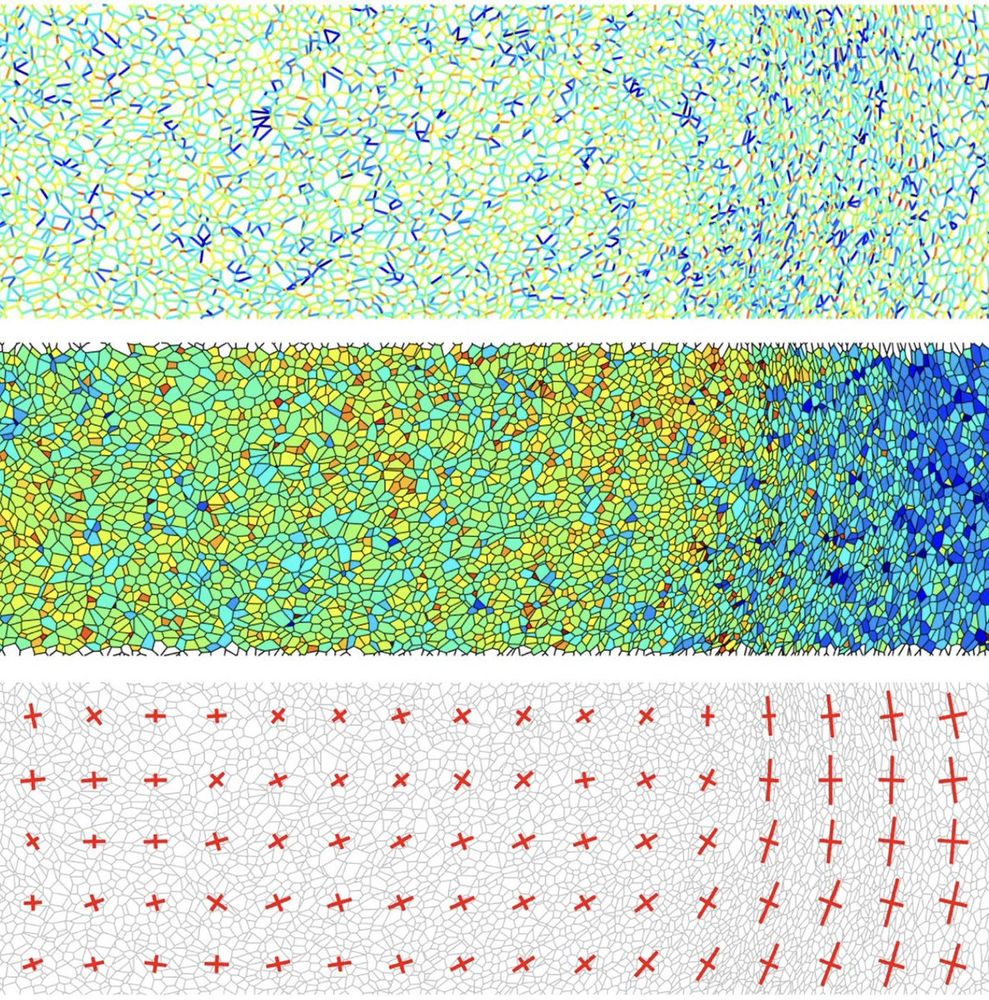
Our code for force inference in epithelial tissues is available online!
Feel free to contact us if you're interested in using it or if you want to collaborate.
Code :Ref :
nature.com/articles/s4159… data.mendeley.com/datasets/78ng4…
Very happy to welcome in our group Alice Gros @_AliceGros as a new Centuri @centuri_ls PhD student on a joint project with Léo Guignard @GuignardLab on morphogenesis of self-organized multicellular systems #Gastruloids
04.10.2021 13:29 — 👍 0 🔁 0 💬 0 📌 0Very grateful to great colleagues and pleased that our roadmap on multi-scale coupling of biochemical and mechanical signals during development is now onlinein IOPPBio @IOPPBio @WarmflashLab
@TimESaunders
@JgrosL
@a_michaut
@EdouardHannezo
@ZevGartner
ow.ly/eRun50EodEL
Very fortunate to welcome today a new postdoc in the group, Valentin Dunsing @DunsingValentin, who will explore/exploit "fluctuations" in morphogenesis.
01.04.2021 06:49 — 👍 1 🔁 0 💬 0 📌 0See our new perspective on cell junction mechanics written together with @Viasnofflab and @JFRupprecht_OM
zpr.io/HW2PN
Check our latest manuscript on the nanoscopic segregation of polarity proteins in epithelia using superresolution. A project led by @PierreMangeol and a great collaboration with Le Bivic team.
biorxiv.org/content/10.110…
Honoured and thrilled to become a EMBO member! A big thank you to all the great members of my team, past and present, and to my inspiring colleagues.
07.07.2020 15:33 — 👍 0 🔁 0 💬 0 📌 0Our first paper on endoderm formation using #gastruloidswith @AMA_Lab
biorxiv.org/content/10.110…

Signez la pétition : La recherche scientifique a besoin d’un plan d’urgencevia @ChangeFrance
chng.it/DjnW7knV

We seek to attract new PIs, computer scientists, physicists, or mathematicians with a theoretical and/or computational biology project, in the Turing Center for Living Systems (CenTuri) in Marseille.
View the offer here
⬇
centuri-livingsystems.org/wp-content/upl…
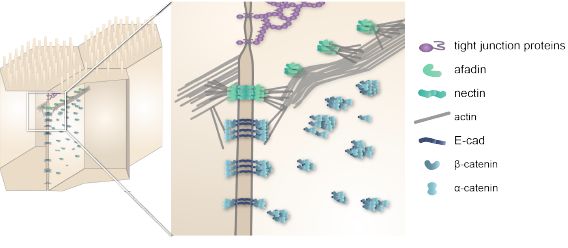
Check our latest manuscript on supramolecular architecture of epithelia:
biorxiv.org/content/10.110…
Last days to register/submit an abstract to our meeting on "Self-organization in multicellular systems" in Cargèse, Corsica, Sept 30 - Oct 4.
centuri-livingsystems.org/csm2019/
Assembly of a persistent apical actin network by the formin Frl/Fmnl tunes epithelial cell deformability
biorxiv.org/content/10.110…
Olga's paper on calcium transients in the Drosophila embryo is in BiorXiv:
disq.us/t/3b8ulq0