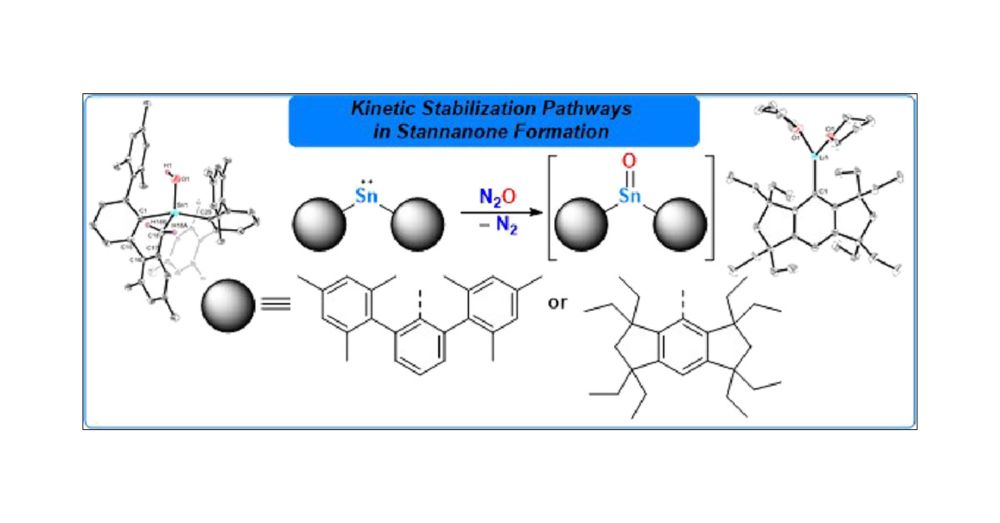
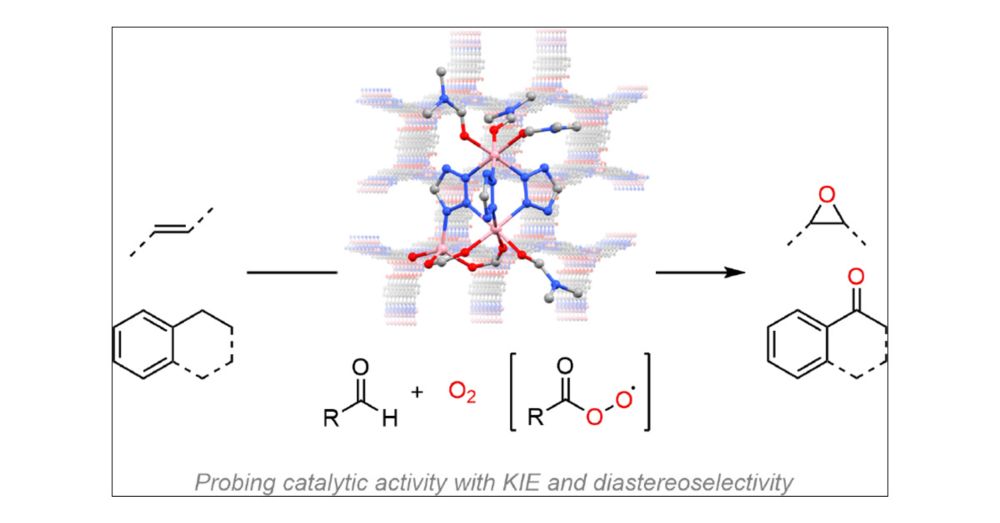
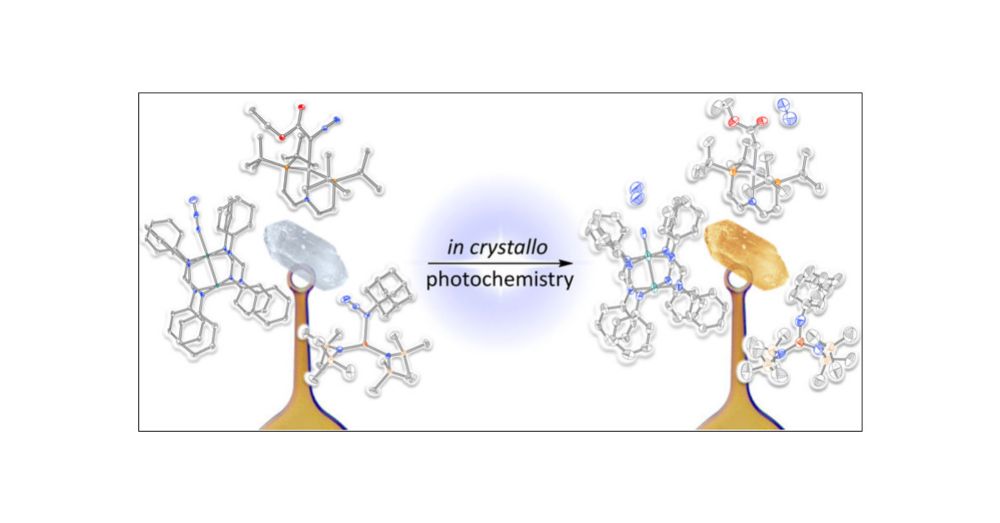
ACS Publications is proud to publish the most trusted, most cited, and most read collection of journals in the chemical and related sciences.
Explore ACS Publications, a division of @acs.org: pubs.acs.org
Updates on the McCrory Lab at the University of Michigan. https://sites.lsa.umich.edu/mccrory-lab
Assistant Professor, UW-Madison Chemistry. Group website: http://martellgroup.chem.wisc.edu.
Prof. of Organic Chemistry⚗️ CATALYSIS & more (membranes, on-surface-chemistry, batteries, data & machine learning). Passionate 👨🔬😀🇪🇺
Associate Professor @UMN. Urgently spreading and learning the OChem Gospel. As a researcher/mentor, I want to (i.) address synthetic challenges associated with CNS disorders and (ii.) invent new reactions of general use to drug discovery.
Assistant Professor at UBC. Inorganic chemistry, electrocatalysis, reaction mechanisms at molecules and electrode interfaces. www.enicholschem.com
Group account. Chemistry with an inorganic flavo(u)r. Views collective. @IUBChemistry
Research Group in the Department of Chemistry at the National University of Singapore (NUS). Transforming chemical & carbohydrate synthesis through base metal catalysis, radical cross-coupling & molecular editing.
The Matson Lab at Virginia Tech Dept of Chemistry develops small molecules and materials for biology, medicine, and sustainability using tools from organic, polymer, and supramolecular chemistry.
Incoming assistant professor at Penn State University, postdoc at Hyster Lab, Ph.D. at Arndtsen Lab
Chemistry professor at Penn State. Associate Editor of ACS Nano. Deputy Editor of ACS Nanoscience Au. Materials chemistry, nanochemistry, solid state chemistry, catalyst discovery.
Inorganic chemistry professor at the University of Illinois Chicago 🧪⚛️⚗️
Associate Editor of Dalton Transactions 📝📔
chemistry professor. catalysis, mechanism, synthesis, and polymers. likes to run.
https://sites.google.com/umn.edu/tonksgroup/home
Professor of Chemistry at University of Basel, Switzerland
Associate professor of chemistry at the University of Minnesota
Engineer/scientist, Nobel Prize in Chemistry 2018
I love evolution, enzymes, protein engineering, AI
Linus Pauling Professor at Caltech
Editor at @science.org, shepherding chemistry papers; views here are my own; he/him