
#membrane seminars are back this THURSDAY for a super session with @dr-downes.bsky.social from the @giuliazanetti.bsky.social lab and Xin Zhou from the Ikonen lab! @felixmendu.bsky.social @ishier.bsky.social
03.12.2025 11:20 — 👍 15 🔁 13 💬 1 📌 0
Last week to submit an abstract to the "Intracellular mechanics and organelle mechanobiology" EMBO workshop #EMBOmechanobio 👇
01.12.2025 08:45 — 👍 5 🔁 3 💬 0 📌 0
It was fun, and a great honour, to receive this award in Barcelona last night. I'm speaking tonight about quantum matters for the City Biennal.
www.biennalciutaticiencia.barcelona/en/activitie...
19.11.2025 16:58 — 👍 21 🔁 1 💬 1 📌 0

Check out our @ascbiology.bsky.social #CellBio2025 Minisymposium on ER Homeostasis. With THIS speaker lineup, this event will be as dynamic, transformative and productive as the ER itself!
So don’t 𝗦𝗧𝗥𝗘𝗦𝗦, 𝙩𝙧𝙖𝙣𝙨𝙡𝙤𝙘𝙖𝙩𝙚 yourselves over and join us to 𝗡𝗘𝗧𝗪𝗢𝗥𝗞 in world of the ER. (All PUNS intended.)
09.11.2025 18:04 — 👍 24 🔁 14 💬 0 📌 1
Join us at @icfo.eu in Barcelona next February for the 1st @embo.org Workshop on "Intracellular mechanics and organelle mechanobiology". I am convinced it will be very interesting! We have extended the abstract submission deadline to December 8, so no excuses :) #EMBOmechanobio
12.11.2025 16:34 — 👍 6 🔁 1 💬 0 📌 0

Intracellular mechanics and organelle mechanobiology
Mechanobiology is an interdisciplinary field that emerges at the cross-section of biology, physics and engineering. It aims to understand how living cells, tissues and animals sense and respond to me…
Announcing a new @embo.org Workshop on "Intracellular mechanics and organelle mechanobiology" that we are organizing with Michael Krieg and Verena Ruprecht at @icfo.eu (Barcelona) on 16-20 February, 2026. Please, repost and spread the word! 🙏 #EMBOmechanobio
meetings.embo.org/event/26-org...
04.07.2025 15:50 — 👍 71 🔁 45 💬 1 📌 5

Imaging Cell Dynamics
11-14 May 2026
Montanyà Hotel, Catalonia, Spain
Register now - early-bird deadline: 16 January 2026
Journal of Cell Science Meeting
Registration is now open to attend Journal of Cell Science's meeting on Imaging Cell Dynamics, organised by Francesca Bottanelli, Guillaume Jacquemet, Michael Way and Giulia Zanetti.
Find out more: biologists.com/meetings/jcs...
#JCSimaging #Microscopy #Microscope #Imaging #Cells #CellScience
09.10.2025 11:15 — 👍 31 🔁 15 💬 0 📌 1

#MembraneTraffickers! Our next online seminar is this Thursday, Nov 6 at 5 PM CEST!
We’ll hear from @agatawitkowska.bsky.social on mechanical control of neurotransmission and @abdourachidthiam.bsky.social on lipid storage mechanisms in health and disease.
Spread the word and join the discussion!
03.11.2025 19:33 — 👍 21 🔁 15 💬 1 📌 0
I have two open positions @ALMU @crg.eu:
1. Imaging Scientist (permanent position. Deadline 11th Nov.) recruitment.crg.eu/content/jobs...
2. Entry-Level Imaging Scientist (12 months fixed-term position. Deadline 18th Nov.) recruitment.crg.eu/content/jobs...
Reposts appreciated.
03.11.2025 14:01 — 👍 5 🔁 11 💬 0 📌 0
Another great opportunity to join us! 👏
#PostDocPosition #AcademicJobs
03.11.2025 14:17 — 👍 1 🔁 1 💬 0 📌 0
📣 We are moving to Barcelona to join the amazing @crg.eu and explore how centrosomes & cilia work, evolve & respond to the environment. We are looking for
PhD students www.crg.eu/en/content/t...
Postdocs gimm.pt/jobs/postdoc...
Other calls to open, also for an experienced lab manager.
Get in touch!
30.10.2025 13:15 — 👍 111 🔁 54 💬 2 📌 3
Reminder: our postdoc and PhD positions are still open. We work across different areas (wet and dry lab), but I am especially after people with a computational biology, bioinformatics, pytorch whisperer kind of background. Protein design and OpenFold/AF also counts.
29.10.2025 06:40 — 👍 5 🔁 3 💬 1 📌 0
MEMBRANE TRAFFICKING | Membrane Trafficking
As usual, more info on the website (which will hopefully be updated soon :)
felixcampelo.wixsite.com/membranetraf...
cc/ @ishier.bsky.social @franbottanelli.bsky.social
27.10.2025 17:02 — 👍 3 🔁 1 💬 0 📌 0

November'25-February'26 membrane trafficking online seminars program
Calling all #Membrane enthusiasts!
Our #MembraneTrafficking online seminar series is back for the 2025-26 season: 8 fantastic speakers from Nov'25 to Feb'26.
The first talks are already next Thursday (Nov 6, 5pm CET) with @agatawitkowska.bsky.social and @abdourachidthiam.bsky.social !
27.10.2025 17:02 — 👍 30 🔁 19 💬 1 📌 2
EVOMG-DN
EvoMG-DN is a European Doctoral Network funded by the Horizon Europe Marie Skłodowska-Curie Actions (MSCA), composed of 14 beneficiaries and associated partners from both academic and non-academic sectors.
🚨🚨🚨
Two super well-funded PhD grants from our MSCA Doctoral Network @evomg-dn.bsky.social at our lab at @melisupf.bsky.social, @crg.eu and the @evomg-bcn.bsky.social Program.
If you're excited about evolution, genomics and biomedical research, this is your DN! 😃
Further info: www.evomg-dn.eu and 👇
15.10.2025 07:02 — 👍 13 🔁 19 💬 1 📌 2
I'm hiring a postdoc! If you're interested in researching mechanisms of 3D tissue morphogenesis and have experience studying cell dynamics and performing quantitative image analysis, consider joining us @mbisg.bsky.social! www.mbi.nus.edu.sg/jobs/seeking...
15.10.2025 01:45 — 👍 9 🔁 10 💬 0 📌 0
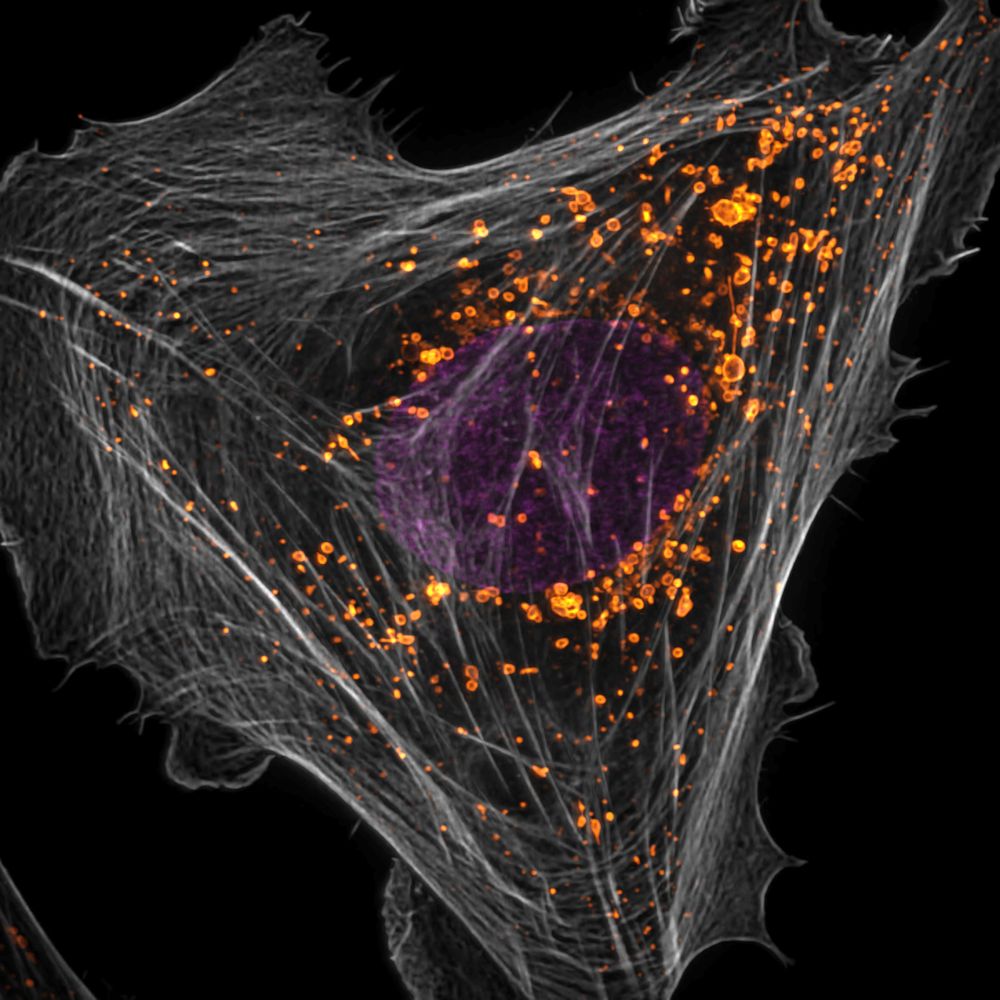

🚨 We’re hiring a postdoc! 🚨
Interested in lysosomes, Parkinson’s disease, cell biology, microscopy — or all of the above? Come work with us at the Bonet-Ponce Lab!
Reach out directly: luis.bonetponce@osumc.edu
Learn more: bonetponcelab.com
Let’s chat!
14.10.2025 17:00 — 👍 32 🔁 19 💬 1 📌 0

We are hiring ! Interested in understanding how neuronal cell diversity arises? Come and join us @upf.edu for a #PhD #FPI fellowship
06.10.2025 12:59 — 👍 10 🔁 11 💬 1 📌 0

September in preprints – Cell biology edition - preLights
Happy October 1st!
A good time to share another #CellBio #preprint reading list put together by a team of preLighters: @sristi.bsky.social, Matthew Davies, @vibhasingh.bsky.social, @fadelvalle.bsky.social & Barbora Knotkova.
Check out their picks ⬇️👀
prelights.biologists.com/prelists/sep...
01.10.2025 11:49 — 👍 6 🔁 4 💬 0 📌 0

Tenure-track Position in Biophysics at Carnegie Mellon University, Department of Physics
Location: Pittsburgh, PA
Open Date: Sep 19, 2025
Description
The Department of Physics at Carnegie Mellon University invites applications for a tenure-track faculty position in biophysics. The appointment is intended to be at the Assistant Professor level, but exceptional candidates at a higher level may also be considered. We seek outstanding candidates with a strong record in cellular and subcellular biophysics. Topics of particular interest include, but are not limited to, uncovering how key characteristics of living systems arise from the interplay between supramolecular cellular structures, how the emergent cellular circuitry defines goals and enables robust decision making, and how metabolic resources are allocated. This encompasses understanding of how information is learned, stored, transduced, and processed across subcellular structures. Applicants with theoretical, data science, or experimental backgrounds within biological physics are encouraged to apply. The ideal candidate will strengthen and extend research programs of current biophysics faculty in the Department of Physics and collaborate with broader life science activities across many departments at CMU and the wider Pittsburgh area.
More details on Interfolio: https://apply.interfolio.com/174360
I am super excited to announce that we have a tenure-track faculty position in biophysics open in the Department of Physics at Carnegie Mellon! 🧪
Interfolio link: apply.interfolio.com/174360
PLEASE, share widely across the blue skies!
Let me briefly explain what we're looking for:
1/10
26.09.2025 15:35 — 👍 98 🔁 89 💬 2 📌 5
intracellular mechanics ! in Barcelona in February (when its freezing anywherelse) !
30.09.2025 19:31 — 👍 6 🔁 2 💬 0 📌 0
Check out this new Roadmap, a fantastic team effort by a consortium of ER researchers. Glad we could provide the space for this synthesis, which came out of discussions at a fruitful meeting, see below:
30.09.2025 07:35 — 👍 12 🔁 8 💬 0 📌 0
It was a great pleasure to work on this paper. What started as a small meeting in January this year resulted in this beautiful piece of work.
30.09.2025 07:36 — 👍 10 🔁 4 💬 0 📌 0
Many thanks Adrien! Indeed, while there are still lots of unknowns (which makes it quite an interesting topic), I believe there is a lot of hidden secretion regulatory layers to be discovered 😃 Any feedback (mechanochemical or not 🤣) will be very much appreciated!
30.09.2025 08:19 — 👍 1 🔁 0 💬 0 📌 0
The Alon lab explores design principles of biological circuits, focusing on systems medicine, aging, and healthspan.
Predoctoral Research Assistant at the Llorca lab @cniostopcancer.bsky.social working on mTORC1 signaling and cryo-EM ❄️🔬. Intrigued by the structural mechanisms of protein assemblies involved in endomembrane signaling and trafficking 🧪.
Leads the Cancer Stem Cells & Metastasis Lab @HMar_research | focused in breast cancer immunotherapy
http://celiaterrassalab.com
Group Leader at EMBL
Searching for PV = nRT in biology
Cell competition in development/gastruloids (postdoc Alfonso Martinez Arias lab)
Previously: adhesion GPCRs in Glioblastoma (PhD Placantonakis Lab NYU)
We study pathogen, cell & tissue mechanobiology in cardiac diseases and cancers ~ Team from Cameroon, Eswatini, Lesotho, South Africa, Togo & Uganda.
mechanobiology.uct.ac.za
PhD Candidate - Bordeleau Lab (Université Laval - QC, Canada)
Cancer Mechanobiology / Alternative splicing ⚙️🔬
FAK, the one FA protein to rule them all 🇨🇦🇲🇫
Google Scholar : https://t.co/kPAuplVlbQ
#MicrobiomeTransmission #MicrobiomeGutBrainAxis
Group leader of the Microbiome Research Group at MELIS-UPF
https://www.upf.edu/web/microbiome
Previously @cibiocm.bsky.social
Evolutionary Biologist, IBE (CSIC-UPF); Department of Medicine and LIfe Sciences, UPF
Neurobiologist 🧠
Leading the Neurobiology of Behavior Research Group (GreNeC - NeuroBio) at the Medicine and Life Sciences Department (MELIS)
Universitat Pompeu Fabra (UPF)
Research professor @UPFBarcelona genetics and regulatory genomics, islet and chromatin but also father, travelling, campervan, sailing, handbike and kayaking.
Associate Professsor @upf.edu. Computational genomics R/Bioc developer. Innate curiosity. Views my own. He/him, vi/vim. Mastodon: @robertclab@genomic.social. Twitter: @robertclab
Principal Investigator at the Synthetic Cell Programming lab, University Pompeu Fabra, Barcelona.
#SynBio / #GeneCircuits / #MicrobiomeEngineering
santosmorenolab.org
Epithelial cell competition. Environment and mutational landscape. Tumor initiation. 3D cultures. Metabolism. Lipid droplets.
https://www.researchgate.net/profile/Albert-Herms
https://orcid.org/0000-0003-2999-8196
Postdoc | GPCRs | membranes | trafficking | signaling | he/him
Single molecule biophysicist, Rosalind Franklin Fellow and Associate Professor at the University of Groningen. Enjoys food, bouldering, silliness & science.
Department and School of Medicine and Life Sciences at @upf.edu. Located at the @prbb.org & member of #SOMM_alliance
www.upf.edu/biomed
We are a research group at University of Freiburg that studies the molecular mechanisms of T cell activation and how to use them for immunotherapy.
https://lillemeier.biologie.uni-freiburg.de/















