
🧪Published my first commentary on RNA vaccines against nonenveloped viruses. www.cell.com/molecular-th...
15.12.2025 15:54 — 👍 9 🔁 2 💬 0 📌 0@jesseerasmus.bsky.social
Director of Virology at HDT Bio. Developing RNA platform for emerging infectious diseases. 🇿🇦🇰🇷

🧪Published my first commentary on RNA vaccines against nonenveloped viruses. www.cell.com/molecular-th...
15.12.2025 15:54 — 👍 9 🔁 2 💬 0 📌 0In my new Nucleic Acid Insights interview, I discuss the evolving science of RNA therapeutics. Addresses safety, manufacturing, and delivery, and how HDT Bio’s LION platform moves beyond LNPs to enable potent, localized, extrahepatic RNA delivery.
www.insights.bio/nucleic-acid...
Excited to announce our new ARPA-H award to “Close the DOORs” mapping how viruses exploit human cells and learning how to shut them. Grateful to @arpa-h.bsky.social and our amazing team!
🔗 www.mountsinai.org/about/newsro...
#ARPAH #Virology #HostPathogen #Innovation #Science

New #NatureComms paper: Muscle-specific gene editing therapy via mammalian fusogen-directed virus-like particles. #Myoblue tinyurl.com/35wcweap
15.10.2025 12:46 — 👍 6 🔁 1 💬 1 📌 0Follow up publication describing immunogenicity and efficacy in NHPs of historical and contemporary H5N1 vaccine compositions against challenge with contemporary cattle virus isolate. What we concluded in mice did not translate to NHPs. www.science.org/doi/10.1126/...
09.10.2025 01:26 — 👍 10 🔁 5 💬 0 📌 0bsky.app/profile/jess...
01.10.2025 00:32 — 👍 0 🔁 0 💬 0 📌 0bsky.app/profile/jess...
01.10.2025 00:31 — 👍 0 🔁 0 💬 1 📌 0Some related posts below. bsky.app/profile/jess...
01.10.2025 00:30 — 👍 0 🔁 0 💬 1 📌 0
Happy to announce that we have initiated a phase I clinical trial to evaluate safety and immunogenicity of our LION/repRNA vaccine against #CCHF. This is the culmination of a great partnership with NIAID, UTMB, and our federal sponsors. More to come! www.prnewswire.com/news-release...
01.10.2025 00:28 — 👍 4 🔁 0 💬 1 📌 0
Seems heads are already rolling.
08.08.2025 20:16 — 👍 0 🔁 0 💬 0 📌 0Even our project, which directly addressed the tolerability and upper respiratory tract immunity gaps highlighted by RFK Jr, was impacted by this decision. They clearly didn’t review the 22 contracts/grants and the data already generated under taxpayer investment. We are 3 yrs into our program…
06.08.2025 02:27 — 👍 3 🔁 0 💬 0 📌 0Excited to meet the D2R community!
11.07.2025 03:07 — 👍 2 🔁 0 💬 0 📌 0Without safety concern but still higher reactogenicity across the board compared to the licensed comparator. Wish there was a way to improve efficacy while retaining the favorable safety profile of the licensed comparator 🤔
02.07.2025 02:06 — 👍 0 🔁 0 💬 0 📌 0Congrats! Super cool finding. Now I want to put gH-UL116-UL141 into our RNA platform and compare to all the other permutations of structural (and now “nonstructural”) antigen candidates.
01.07.2025 14:32 — 👍 2 🔁 0 💬 1 📌 0
🧪 New paper from our team at HDT Bio highlighting that combo repRNA vaccines can lead to excessive innate activation that ultimately inhibits vaccine effectiveness in NHPs. Use of repRNA formulations with restricted biodistribution can alleviate systemic innate response and restore effectiveness.
19.06.2025 04:08 — 👍 5 🔁 0 💬 0 📌 0No I haven’t. I’ll check it out!
19.06.2025 01:36 — 👍 1 🔁 0 💬 0 📌 0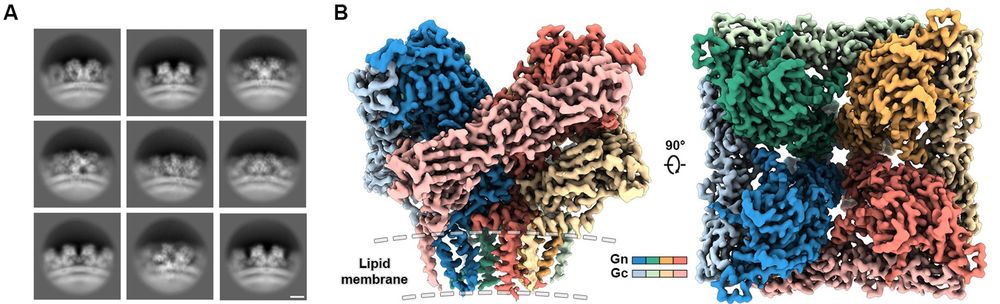
Our latest preprint is now available doi.org/10.1101/2025...! Led by my postdoc, Luqiang Guo @luqiang.bsky.social, we determined a ~2.4 A resolution structure of the hantavirus glycoprotein tetramer on the surface of VLPs. Also determined structures of dimers of tetramers and Fab-bound tetramers.
18.06.2025 14:24 — 👍 69 🔁 23 💬 2 📌 2I didn’t realize you could filter the posts of who you follow! The paper feed is just what I was looking for.
13.06.2025 14:44 — 👍 3 🔁 2 💬 1 📌 0Shook Nelson Mandela’s hand when I was 10 years old at some kind of gathering in a community park. Just remember him getting off a helicopter and walking through a crowd shaking hands, including mine.
13.06.2025 14:01 — 👍 0 🔁 0 💬 0 📌 0Outstanding op-ed from Carole LaBonne
Universities and the government: Which needs the other more?
College-based research labs are the workhorses of national progress and prosperity.
wapo.st/3ZR1vFm
1/n
Nothing published on this yet. But we are working on it!
21.05.2025 11:47 — 👍 2 🔁 0 💬 0 📌 0Summary:
1) LION/repRNA (don’t confuse with LNP/mRNA) can indeed confer protection on par with the VSV platform (at least in guinea pigs) after one shot.
2) LION & VSV elicited similar IgG titers but with a unique effector profile. LION induced higher ADCP & ADNP activity but lower ADCD. (2/2)

Collab btwn NIAID (Dr. Andrea Marzi’s lab) and HDT Bio just published! 🧪
Filovirus gold-standard VSV vax platform compared to LION/repRNA (each encoding same antigen seq) for ability to provide single-dose protection in guinea pigs against Sudan virus challenge 🧵(1/2) www.nature.com/articles/s41...
Mice were protected from death and viremia (panels d and e) in the inactivated vaccine group but not from morbidity (panels b and c), suggesting some nuance in the quality of immune response between authentic and inactivated versions. Lots of potential explanations out there.
04.05.2025 16:14 — 👍 1 🔁 0 💬 0 📌 0
In fig 5 of the 🔗 paper, I compared the durability of protective immunity elicited by same doses of the exact same virus/vaccine (non replicating). One was inactivated with formalin (open circles); the other was not (closed circles). Inactivation killed durability. www.nature.com/articles/nm....
04.05.2025 16:09 — 👍 5 🔁 1 💬 1 📌 0I noticed this statement from NIH regarding durability of the inactivated approach. I didn’t see any durability data in the published studies of this candidate. Anyone aware of durability studies performed using inactivated virus platforms? I did one experiment in grad school (in thread below).
04.05.2025 16:09 — 👍 9 🔁 2 💬 1 📌 0
Don’t get me wrong, I’m appalled by what this administration is doing to science. But I think there is something else going on here. Whatever happened will undoubtedly get politicized though.
01.05.2025 01:54 — 👍 0 🔁 0 💬 2 📌 0
Just published! Here we report immunogenicity and efficacy of our replicon RNA vaccine against Crimean-Congo hemorrhagic fever in a cynomolgus macaque model of #CCHFV infection. Stay tuned as we advance this candidate in clinical trials.
www.thelancet.com/journals/ebi...
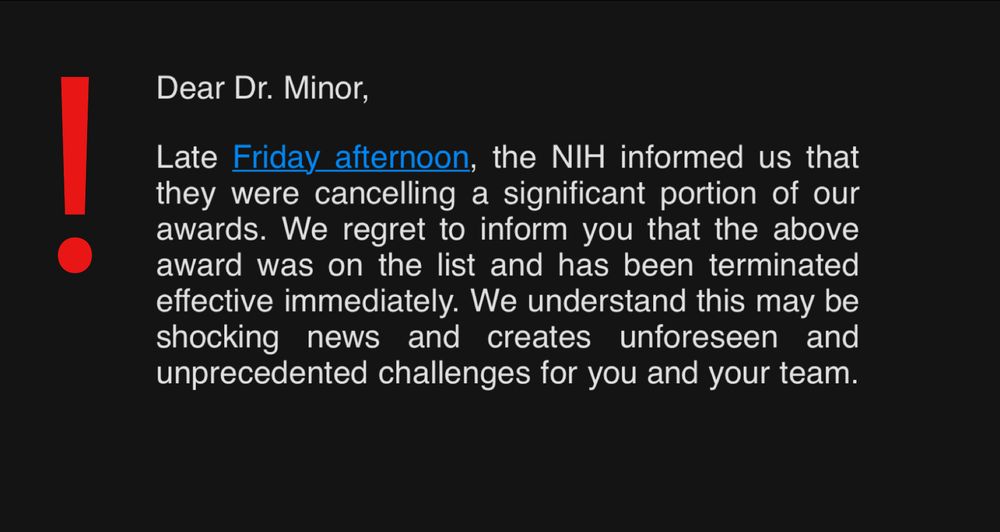
There are days in life that shake you.
I’m shattered 💔 to share that I just found out that the US Government terminated my 2024 NIH Director’s Early Independence Award (~$2 million), threatening my long-promised assistant professor job at Columbia University
& academic career... 1/🧵
Could you link to the first one please?🙏
18.03.2025 09:59 — 👍 2 🔁 0 💬 2 📌 0