
How long until someone uses these to degrade BRD4?!
17.09.2025 23:54 — 👍 9 🔁 1 💬 0 📌 0@svinograd.bsky.social
PhD Student at UC Irvine | Pharmacological Sciences

How long until someone uses these to degrade BRD4?!
17.09.2025 23:54 — 👍 9 🔁 1 💬 0 📌 0
While disease treatments often inhibit proteins, #PROTAC degrade them via ubiquitinase-protease recruitment. Darci Trader @ucirvine.bsky.social now show in J Med Cem proteases can be recruitet without ubiquitinases — a still less potent but interesting alternative.
pubs.acs.org/doi/10.1021/...
Very exciting @traderlab.bsky.social
28.04.2025 17:33 — 👍 1 🔁 0 💬 0 📌 0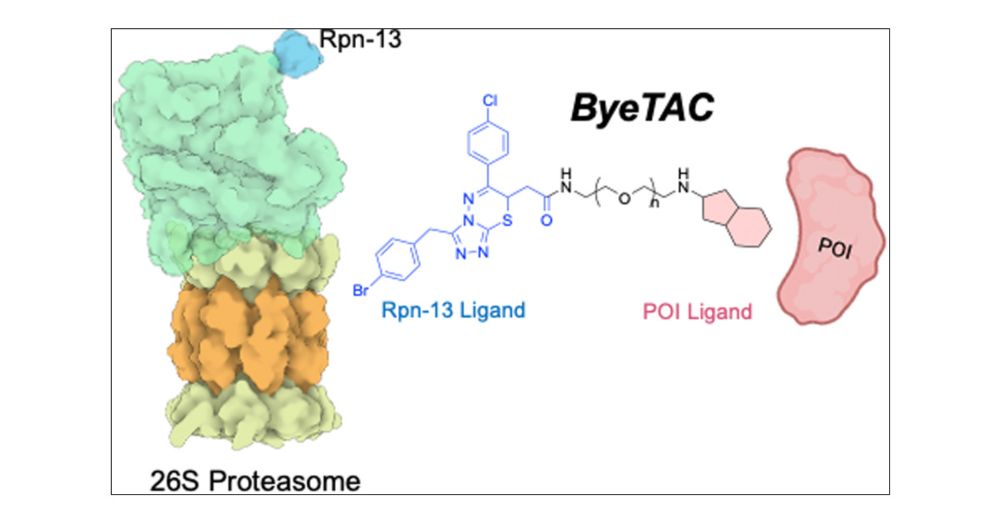
ByeTACs is finally out in J. Med Chem. Congrats team! Can't wait to see how else we can use this degradation technology. @codyloy.bsky.social
pubs.acs.org/doi/10.1021/...

Beyond thrilled to finally have this paper out in J. Med. Chem. Check out @traderlab.bsky.social new targeted protein degradation mechanism, ByeTAC. 🧪🥼
doi.org/10.1021/acs....
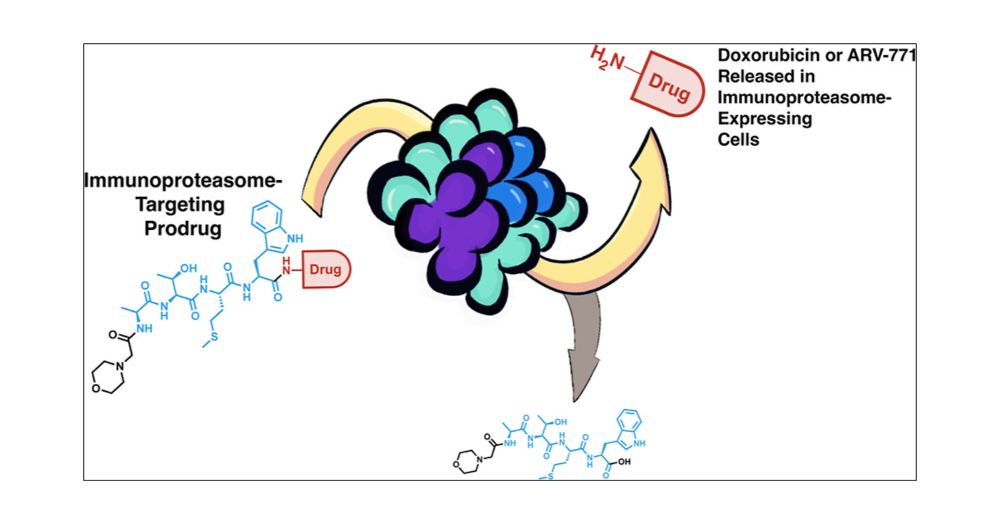
Really proud to have this out in J Med Chem! Congrats to Christine and @codyloy.bsky.social
pubs.acs.org/doi/10.1021/...
Starting 2025 off with a review about degrons in @rscmedchem.bsky.social. Congrats Tim!
pubs.rsc.org/en/content/a...